Basal Ganglia Neuromodulation Over Multiple Temporal and Structural Scales-Simulations of Direct Pathway MSNs Investigate the Fast Onset of Dopaminergic Effects and Predict the Role of Kv4.2
- PMID: 29467627
- PMCID: PMC5808142
- DOI: 10.3389/fncir.2018.00003
Basal Ganglia Neuromodulation Over Multiple Temporal and Structural Scales-Simulations of Direct Pathway MSNs Investigate the Fast Onset of Dopaminergic Effects and Predict the Role of Kv4.2
Abstract
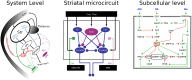
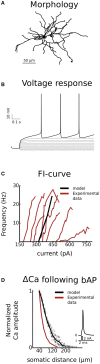
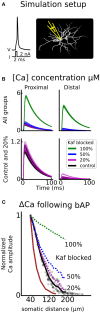
The basal ganglia are involved in the motivational and habitual control of motor and cognitive behaviors. Striatum, the largest basal ganglia input stage, integrates cortical and thalamic inputs in functionally segregated cortico-basal ganglia-thalamic loops, and in addition the basal ganglia output nuclei control targets in the brainstem. Striatal function depends on the balance between the direct pathway medium spiny neurons (D1-MSNs) that express D1 dopamine receptors and the indirect pathway MSNs that express D2 dopamine receptors. The striatal microstructure is also divided into striosomes and matrix compartments, based on the differential expression of several proteins. Dopaminergic afferents from the midbrain and local cholinergic interneurons play crucial roles for basal ganglia function, and striatal signaling via the striosomes in turn regulates the midbrain dopaminergic system directly and via the lateral habenula. Consequently, abnormal functions of the basal ganglia neuromodulatory system underlie many neurological and psychiatric disorders. Neuromodulation acts on multiple structural levels, ranging from the subcellular level to behavior, both in health and disease. For example, neuromodulation affects membrane excitability and controls synaptic plasticity and thus learning in the basal ganglia. However, it is not clear on what time scales these different effects are implemented. Phosphorylation of ion channels and the resulting membrane effects are typically studied over minutes while it has been shown that neuromodulation can affect behavior within a few hundred milliseconds. So how do these seemingly contradictory effects fit together? Here we first briefly review neuromodulation of the basal ganglia, with a focus on dopamine. We furthermore use biophysically detailed multi-compartmental models to integrate experimental data regarding dopaminergic effects on individual membrane conductances with the aim to explain the resulting cellular level dopaminergic effects. In particular we predict dopaminergic effects on Kv4.2 in D1-MSNs. Finally, we also explore dynamical aspects of the onset of neuromodulation effects in multi-scale computational models combining biochemical signaling cascades and multi-compartmental neuron models.
Keywords: Kv4.2; dopamine; kinetic modeling; medium spiny projection neurons; simulations; striatum; subcellular signaling.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

