Brivaracetam in the Treatment of Patients with Epilepsy-First Clinical Experiences
- PMID: 29467714
- PMCID: PMC5808159
- DOI: 10.3389/fneur.2018.00038
Brivaracetam in the Treatment of Patients with Epilepsy-First Clinical Experiences
Abstract
Objectives: To assess first clinical experiences with brivaracetam (BRV) in the treatment of epilepsies.
Methods: Data on patients treated with BRV from February to December 2016 and with at least one clinical follow-up were collected from electronic patient records. Data on safety and efficacy were evaluated retrospectively.
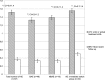
Results: In total, 93 patients were analyzed; 12 (12.9%) received BRV in monotherapy. The mean duration to follow-up was 4.85 months (MD = 4 months; SD = 3.63). Fifty-seven patients had more than one seizure per month at baseline and had a follow-up of more than 4 weeks; the rate of ≥50% responders was 35.1% (n = 20) in this group, of which five (8.8%) patients were newly seizure-free. In 50.5% (47/93), patients were switched from levetiracetam (LEV) to BRV, of which 43 (46.2%) were switched immediately. Adverse events (AE) occurred in 39.8%, with 22.6% experiencing behavioral and 25.8% experiencing non-behavioral AE. LEV-related AE (LEV-AE) were significantly reduced by switching to BRV. The discontinuation of BRV was reported in 26/93 patients (28%); 10 of those were switched back to LEV with an observed reduction of AE in 70%. For clinical reasons, 12 patients received BRV in monotherapy, 75% were seizure-free, and previous LEV-AE improved in 6/9 patients. BRV-related AE occurred in 5/12 cases, and five patients discontinued BRV.
Conclusion: BRV seems to be a safe, easy, and effective option in the treatment of patients with epilepsy, especially in the treatment of patients who have psychiatric comorbidities and might not be good candidates for LEV treatment. BRV broadens the therapeutic spectrum and facilitates personalized treatment.
Keywords: brivaracetam; epilepsy; levetiracetam; side effects; treatment.
Figures

Similar articles
-
Effectiveness and Tolerability of 12-Month Brivaracetam in the Real World: EXPERIENCE, an International Pooled Analysis of Individual Patient Records.CNS Drugs. 2023 Sep;37(9):819-835. doi: 10.1007/s40263-023-01033-4. Epub 2023 Sep 9. CNS Drugs. 2023. PMID: 37684497 Free PMC article. Review.
-
Brivaracetam for the treatment of refractory epilepsy in patients with prior exposure to levetiracetam: A retrospective outcome analysis.Seizure. 2022 Mar;96:102-107. doi: 10.1016/j.seizure.2022.02.007. Epub 2022 Feb 12. Seizure. 2022. PMID: 35184005
-
Tolerability, efficacy and retention rate of Brivaracetam in patients previously treated with Levetiracetam: A monocenter retrospective outcome analysis.Seizure. 2018 Oct;61:98-103. doi: 10.1016/j.seizure.2018.07.017. Epub 2018 Jul 29. Seizure. 2018. PMID: 30118932
-
Retention of brivaracetam in adults with drug-resistant epilepsy at a single tertiary care center.Epilepsy Behav. 2022 Oct;135:108868. doi: 10.1016/j.yebeh.2022.108868. Epub 2022 Aug 16. Epilepsy Behav. 2022. PMID: 35985166
-
Brivaracetam efficacy and safety in focal epilepsy.Expert Rev Neurother. 2019 Oct;19(10):955-964. doi: 10.1080/14737175.2019.1631160. Epub 2019 Jun 24. Expert Rev Neurother. 2019. PMID: 31195850 Review.
Cited by
-
A prospective, observational, multicentre study to evaluate the efficacy of brivaracetam as adjuvant therapy for epilepsy: The Bravo study.Drugs Context. 2024 Jul 8;13:2024-3-2. doi: 10.7573/dic.2024-3-2. eCollection 2024. Drugs Context. 2024. PMID: 38989131 Free PMC article.
-
First clinical postmarketing experiences in the treatment of epilepsies with brivaracetam: a retrospective observational multicentre study.BMJ Open. 2019 Nov 4;9(11):e030746. doi: 10.1136/bmjopen-2019-030746. BMJ Open. 2019. PMID: 31690606 Free PMC article.
-
Effectiveness and Tolerability of 12-Month Brivaracetam in the Real World: EXPERIENCE, an International Pooled Analysis of Individual Patient Records.CNS Drugs. 2023 Sep;37(9):819-835. doi: 10.1007/s40263-023-01033-4. Epub 2023 Sep 9. CNS Drugs. 2023. PMID: 37684497 Free PMC article. Review.
-
Levetiracetam and brivaracetam: a review of evidence from clinical trials and clinical experience.Ther Adv Neurol Disord. 2019 Sep 9;12:1756286419873518. doi: 10.1177/1756286419873518. eCollection 2019. Ther Adv Neurol Disord. 2019. PMID: 31523280 Free PMC article. Review.
-
Current Role of Brivaracetam in the Management of Epilepsy in Adults and Children: A Systematic Review.Cureus. 2024 Nov 10;16(11):e73413. doi: 10.7759/cureus.73413. eCollection 2024 Nov. Cureus. 2024. PMID: 39664134 Free PMC article. Review.
References
-
- Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al. Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation. Health Technol Assess (2005) 9(15):1–157. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

