Cytokine Networks between Innate Lymphoid Cells and Myeloid Cells
- PMID: 29467768
- PMCID: PMC5808287
- DOI: 10.3389/fimmu.2018.00191
Cytokine Networks between Innate Lymphoid Cells and Myeloid Cells
Abstract
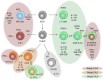
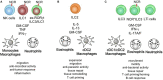
Innate lymphoid cells (ILCs) are an essential component of the innate immune system in vertebrates. They are developmentally rooted in the lymphoid lineage and can diverge into at least three transcriptionally distinct lineages. ILCs seed both lymphoid and non-lymphoid tissues and are locally self-maintained in tissue-resident pools. Tissue-resident ILCs execute important effector functions making them key regulator in tissue homeostasis, repair, remodeling, microbial defense, and anti-tumor immunity. Similar to T lymphocytes, ILCs possess only few sensory elements for the recognition of non-self and thus depend on extrinsic cellular sensory elements residing within the tissue. Myeloid cells, including mononuclear phagocytes (MNPs), are key sentinels of the tissue and are able to translate environmental cues into an effector profile that instructs lymphocyte responses. The adaptation of myeloid cells to the tissue state thus influences the effector program of ILCs and serves as an example of how environmental signals are integrated into the function of ILCs via a tissue-resident immune cell cross talks. This review summarizes our current knowledge on the role of myeloid cells in regulating ILC functions and discusses how feedback communication between ILCs and myeloid cells contribute to stabilize immune homeostasis in order to maintain the healthy state of an organ.
Keywords: Group 1 ILCs; ILC2; ILC3; cytokines; dendritic cells; innate lymphoid cells; macrophages; myeloid cells.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

