Female mate choice of male signals is unlikely to promote ecological adaptation in Enchenopa treehoppers (Hemiptera: Membracidae)
- PMID: 29468032
- PMCID: PMC5817129
- DOI: 10.1002/ece3.3817
Female mate choice of male signals is unlikely to promote ecological adaptation in Enchenopa treehoppers (Hemiptera: Membracidae)
Abstract
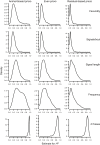
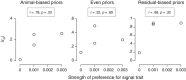
A key question in speciation research is how ecological and sexual divergence arise and interact. We tested the hypothesis that mate choice causes local adaptation and ecological divergence using the rationale that the performance~signal trait relationship should parallel the attractiveness~signal trait relationship. We used female fecundity as a measure of ecological performance. We used a species in the Enchenopa binotata treehopper complex, wherein speciation involves adaptation to novel environments and divergence in sexual communication. We used a full-sibling, split-family rearing design to estimate genetic correlations (rG) between fecundity and signal traits, and compared those relationships against population-level mate preferences for the signal traits. Animal model estimates for rG between female fecundity and male signal traits overlapped zero-rejecting the hypothesis-but could reflect sample size limitations. The magnitude of rG correlated with the strength of the mate preferences for the corresponding signal traits, especially for signal frequency, which has the strongest mate preference and the most divergence in the complex. However, signal frequencies favored by the population-level mate preference are not associated with high fecundity. Therefore, mate preferences do not appear to have been selected to favor high-performance genotypes. Our findings suggest that ecological and sexual divergence may arise separately, but reinforce each other, during speciation.
Keywords: adaptation; ecological speciation; vibrational signal.
Figures





References
-
- Andersson, M. (1994). Sexual selection. Princeton, NJ: Princeton University Press.
-
- Bondurianky, R. , & Rowe, L. (2005). Sexual selection, genetic architecture, and the condition dependence of body shape in the sexually dimorphic fly Prochyliza xanthosoma (Piophilidae). Evolution, 59, 138–151. https://doi.org/10.1111/j.0014-3820.2005.tb00901.x - DOI - PubMed
-
- Bonduriansky, R. (2007). Sexual selection and allometry: A critical reappraisal of the evidence and ideas. Evolution, 61, 838–849. https://doi.org/10.1111/j.1558-5646.2007.00081.x - DOI - PubMed
-
- Boul, K. E. , Funk, W. C. , Darst, C. R. , Cannatella, D. C. , & Ryan, M. J. (2007). Sexual selection drives speciation in an Amazonian frog. Proceedings of the Royal Society of London B: Biological Sciences, 274, 399–406. https://doi.org/10.1098/rspb.2006.3736 - DOI - PMC - PubMed
-
- Byers, J. A. , Hebets, E. , & Podos, J. (2010). Female mate choice based upon male motor performance. Animal Behavior, 79, 771–778. https://doi.org/10.1016/j.anbehav.2010.01.009 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

