Enhancing muconic acid production from glucose and lignin-derived aromatic compounds via increased protocatechuate decarboxylase activity
- PMID: 29468118
- PMCID: PMC5779730
- DOI: 10.1016/j.meteno.2016.04.002
Enhancing muconic acid production from glucose and lignin-derived aromatic compounds via increased protocatechuate decarboxylase activity
Abstract
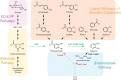
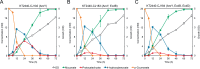
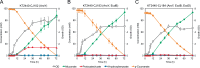
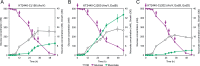
The conversion of biomass-derived sugars and aromatic molecules to cis,cis-muconic acid (referred to hereafter as muconic acid or muconate) has been of recent interest owing to its facile conversion to adipic acid, an important commodity chemical. Metabolic routes to produce muconate from both sugars and many lignin-derived aromatic compounds require the use of a decarboxylase to convert protocatechuate (PCA, 3,4-dihydroxybenzoate) to catechol (1,2-dihydroxybenzene), two central aromatic intermediates in this pathway. Several studies have identified the PCA decarboxylase as a metabolic bottleneck, causing an accumulation of PCA that subsequently reduces muconate production. A recent study showed that activity of the PCA decarboxylase is enhanced by co-expression of two genetically associated proteins, one of which likely produces a flavin-derived cofactor utilized by the decarboxylase. Using entirely genome-integrated gene expression, we have engineered Pseudomonas putida KT2440-derived strains to produce muconate from either aromatic molecules or sugars and demonstrate in both cases that co-expression of these decarboxylase associated proteins reduces PCA accumulation and enhances muconate production relative to strains expressing the PCA decarboxylase alone. In bioreactor experiments, co-expression increased the specific productivity (mg/g cells/h) of muconate from the aromatic lignin monomer p-coumarate by 50% and resulted in a titer of >15 g/L. In strains engineered to produce muconate from glucose, co-expression more than tripled the titer, yield, productivity, and specific productivity, with the best strain producing 4.92±0.48 g/L muconate. This study demonstrates that overcoming the PCA decarboxylase bottleneck can increase muconate yields from biomass-derived sugars and aromatic molecules in industrially relevant strains and cultivation conditions.
Keywords: Lignin valorization; Muconic acid; Protocatechuate decarboxylase; Pseudomonas Putida KT2440; cis,cis-Muconate.
Figures

Similar articles
-
Engineering glucose metabolism for enhanced muconic acid production in Pseudomonas putida KT2440.Metab Eng. 2020 May;59:64-75. doi: 10.1016/j.ymben.2020.01.001. Epub 2020 Jan 10. Metab Eng. 2020. PMID: 31931111
-
Debottlenecking 4-hydroxybenzoate hydroxylation in Pseudomonas putida KT2440 improves muconate productivity from p-coumarate.Metab Eng. 2022 Mar;70:31-42. doi: 10.1016/j.ymben.2021.12.010. Epub 2022 Jan 1. Metab Eng. 2022. PMID: 34982998
-
Comparison of wild-type KT2440 and genome-reduced EM42 Pseudomonas putida strains for muconate production from aromatic compounds and glucose.Metab Eng. 2024 Jan;81:88-99. doi: 10.1016/j.ymben.2023.11.004. Epub 2023 Nov 23. Metab Eng. 2024. PMID: 38000549
-
Lignin valorization by bacterial genus Pseudomonas: State-of-the-art review and prospects.Bioresour Technol. 2021 Jan;320(Pt B):124412. doi: 10.1016/j.biortech.2020.124412. Epub 2020 Nov 16. Bioresour Technol. 2021. PMID: 33249259 Review.
-
Lignin valorization using biological approach.Biotechnol Appl Biochem. 2021 Jun;68(3):459-468. doi: 10.1002/bab.1995. Epub 2020 Aug 13. Biotechnol Appl Biochem. 2021. PMID: 32725827 Review.
Cited by
-
Strategies for the production of biochemicals in bioenergy crops.Biotechnol Biofuels. 2020 Apr 15;13:71. doi: 10.1186/s13068-020-01707-x. eCollection 2020. Biotechnol Biofuels. 2020. PMID: 32318116 Free PMC article. Review.
-
Characterization of alkylguaiacol-degrading cytochromes P450 for the biocatalytic valorization of lignin.Proc Natl Acad Sci U S A. 2020 Oct 13;117(41):25771-25778. doi: 10.1073/pnas.1916349117. Epub 2020 Sep 28. Proc Natl Acad Sci U S A. 2020. PMID: 32989155 Free PMC article.
-
Industrial biotechnology of Pseudomonas putida: advances and prospects.Appl Microbiol Biotechnol. 2020 Sep;104(18):7745-7766. doi: 10.1007/s00253-020-10811-9. Epub 2020 Aug 13. Appl Microbiol Biotechnol. 2020. PMID: 32789744 Free PMC article. Review.
-
Blue valorization of lignin-derived monomers via reprogramming marine bacterium Roseovarius nubinhibens.Appl Environ Microbiol. 2024 Jul 24;90(7):e0089024. doi: 10.1128/aem.00890-24. Epub 2024 Jun 28. Appl Environ Microbiol. 2024. PMID: 38940564 Free PMC article.
-
A Novel Gene Cluster Is Involved in the Degradation of Lignin-Derived Monoaromatics in Thermus oshimai JL-2.Appl Environ Microbiol. 2021 May 11;87(11):e01589-20. doi: 10.1128/AEM.01589-20. Print 2021 May 11. Appl Environ Microbiol. 2021. PMID: 33741620 Free PMC article.
References
-
- Beckham G.T., Johnson C.W., Karp E.M., Salvachúa D., Vardon D.R. Opportunities and challenges in biological lignin valorization. Curr. Opin. Biotechnol. 2016;42:40–53. - PubMed
-
- Belda E., van Heck R.G.A., Lopez-Sanchez M.J., Cruveiller S., Barbe V., Fraser C., Klenk H.-P., Petersen J., Morgat A., Nikel P.I., Vallenet D., Rouy Z., Sekowska A., Martins dos Santos V.A.P., de Lorenzo V., Danchin A., Médigue C. The revisited genome of Pseudomonas putida KT2440 enlightens its value as a robust metabolic chassis. Environ. Microbiol. 2016 - PubMed
-
- Chavarría M., Nikel P.I., Pérez-Pantoja D., de Lorenzo V. The Entner-Doudoroff pathway empowers Pseudomonas putida KT2440 with a high tolerance to oxidative stress. Environ. Microbiol. 2013;15:1772–1785. - PubMed
-
- Choi K.-H., Kumar A., Schweizer H.P. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: Application for DNA fragment transfer between chromosomes and plasmid transformation. J. Microbiol. Methods. 2006;64:391–397. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

