Role of endoplasmic reticulum stress in 12/15-lipoxygenase-induced retinal microvascular dysfunction in a mouse model of diabetic retinopathy
- PMID: 29468369
- PMCID: PMC5878142
- DOI: 10.1007/s00125-018-4560-z
Role of endoplasmic reticulum stress in 12/15-lipoxygenase-induced retinal microvascular dysfunction in a mouse model of diabetic retinopathy
Abstract
Aims/hypothesis: Our earlier studies have established the role of 12/15-lipoxygenase (LO) in mediating the inflammatory reaction in diabetic retinopathy. However, the exact mechanism is still unclear. The goal of the current study was to identify the potential role of endoplasmic reticulum (ER) stress as a major cellular stress response in the 12/15-LO-induced retinal changes in diabetic retinopathy.
Methods: We used in vivo and in vitro approaches. For in vivo studies, experimental diabetes was induced in wild-type (WT) mice and 12/15-Lo (also known as Alox15) knockout mice (12/15-Lo-/-); ER stress was then evaluated after 12-14 weeks of diabetes. We also tested the effect of intravitreal injection of 12-hydroxyeicosatetraenoic acid (HETE) on retinal ER stress in WT mice and in mice lacking the catalytic subunit of NADPH oxidase, encoded by Nox2 (also known as Cybb) (Nox2-/- mice). In vitro studies were performed using human retinal endothelial cells (HRECs) treated with 15-HETE (0.1 μmol/l) or vehicle, with or without ER stress or NADPH oxidase inhibitors. This was followed by evaluation of ER stress response, NADPH oxidase expression/activity and the levels of phosphorylated vascular endothelial growth factor receptor-2 (p-VEGFR2) by western blotting and immunoprecipitation assays. Moreover, real-time imaging of intracellular calcium (Ca2+) release in HRECs treated with or without 15-HETE was performed using confocal microscopy.
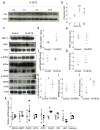
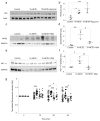
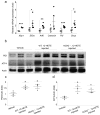
Results: Deletion of 12/15-Lo significantly attenuated diabetes-induced ER stress in mouse retina. In vitro, 15-HETE upregulated ER stress markers such as phosphorylated RNA-dependent protein kinase-like ER-regulated kinase (p-PERK), activating transcription factor 6 (ATF6) and protein disulfide isomerase (PDI) in HRECs. Inhibition of ER stress reduced 15-HETE-induced-leucocyte adhesion, VEGFR2 phosphorylation and NADPH oxidase expression/activity. However, inhibition of NADPH oxidase or deletion of Nox2 had no effect on ER stress induced by the 12/15-LO-derived metabolites both in vitro and in vivo. We also found that 15-HETE increases the intracellular calcium in HRECs.
Conclusions/interpretation: ER stress contributes to 12/15-LO-induced retinal inflammation in diabetic retinopathy via activation of NADPH oxidase and VEGFR2. Perturbation of calcium homeostasis in the retina might also play a role in linking 12/15-LO to retinal ER stress and subsequent microvascular dysfunction in diabetic retinopathy.
Keywords: 12-HETE; 12/15-Lipoxygenase; 15-HETE; Bioactive lipids; Calcium; Diabetic retinopathy; ER stress; Eicosanoids; NADPH oxidase; VEGFR2.
Conflict of interest statement
Figures



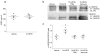

Similar articles
-
Pigment epithelium-derived factor inhibits retinal microvascular dysfunction induced by 12/15-lipoxygenase-derived eicosanoids.Biochim Biophys Acta. 2015 Mar;1851(3):290-8. doi: 10.1016/j.bbalip.2014.12.017. Epub 2015 Jan 3. Biochim Biophys Acta. 2015. PMID: 25562624 Free PMC article.
-
A lipidomic screen of hyperglycemia-treated HRECs links 12/15-Lipoxygenase to microvascular dysfunction during diabetic retinopathy via NADPH oxidase.J Lipid Res. 2015 Mar;56(3):599-611. doi: 10.1194/jlr.M056069. Epub 2015 Jan 17. J Lipid Res. 2015. PMID: 25598081 Free PMC article.
-
Targeting of 12/15-Lipoxygenase in retinal endothelial cells, but not in monocytes/macrophages, attenuates high glucose-induced retinal leukostasis.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Jun;1862(6):636-645. doi: 10.1016/j.bbalip.2017.03.010. Epub 2017 Mar 27. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 28351645 Free PMC article.
-
Key Role of 12-Lipoxygenase and Its Metabolite 12-Hydroxyeicosatetraenoic Acid (12-HETE) in Diabetic Retinopathy.Curr Eye Res. 2022 Mar;47(3):329-335. doi: 10.1080/02713683.2021.1995003. Epub 2022 Feb 7. Curr Eye Res. 2022. PMID: 35129022 Review.
-
Nox NADPH oxidases and the endoplasmic reticulum.Antioxid Redox Signal. 2014 Jun 10;20(17):2755-75. doi: 10.1089/ars.2013.5605. Epub 2014 Feb 26. Antioxid Redox Signal. 2014. PMID: 24386930 Free PMC article. Review.
Cited by
-
GLCCI1 alleviates GRP78-initiated endoplasmic reticulum stress-induced apoptosis of retinal ganglion cells in diabetic retinopathy by upregulating and interacting with HSP90AB1.Sci Rep. 2024 Nov 4;14(1):26665. doi: 10.1038/s41598-024-75874-4. Sci Rep. 2024. PMID: 39496608 Free PMC article.
-
Hyperhomocysteinemia dysregulates plasma levels of polyunsaturated fatty acids-derived eicosanoids.Life Res (Auckl). 2022 Apr;5(2):14. doi: 10.53388/2022-0106-103. Life Res (Auckl). 2022. PMID: 36341141 Free PMC article.
-
Aldehyde Dehydrogenase and Aldo-Keto Reductase Enzymes: Basic Concepts and Emerging Roles in Diabetic Retinopathy.Antioxidants (Basel). 2023 Jul 21;12(7):1466. doi: 10.3390/antiox12071466. Antioxidants (Basel). 2023. PMID: 37508004 Free PMC article. Review.
-
A Hypothesis From Metabolomics Analysis of Diabetic Retinopathy: Arginine-Creatine Metabolic Pathway May Be a New Treatment Strategy for Diabetic Retinopathy.Front Endocrinol (Lausanne). 2022 Mar 24;13:858012. doi: 10.3389/fendo.2022.858012. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35399942 Free PMC article. Review.
-
Synthesis of new multitarget-directed ligands containing thienopyrimidine nucleus for inhibition of 15-lipoxygenase, cyclooxygenases, and pro-inflammatory cytokines.Eur J Med Chem. 2023 Aug 5;256:115443. doi: 10.1016/j.ejmech.2023.115443. Epub 2023 May 5. Eur J Med Chem. 2023. PMID: 37182334 Free PMC article.
References
-
- Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290:2057–2060. - PubMed
-
- Leal EC, Manivannan A, Hosoya K, et al. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2007;48:5257–5265. - PubMed
-
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. - PubMed
-
- Bai N, Tang S, Ma J, Luo Y, Lin S. Increased expression of intercellular adhesion molecule-1, vascular cellular adhesion molecule-1 and leukocyte common antigen in diabetic rat retina. Yan Ke Xue Bao. 2003;19:176–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

