Neurobiological Correlates of Alpha-Tocopherol Antiepileptogenic Effects and MicroRNA Expression Modulation in a Rat Model of Kainate-Induced Seizures
- PMID: 29468563
- PMCID: PMC6132771
- DOI: 10.1007/s12035-018-0946-7
Neurobiological Correlates of Alpha-Tocopherol Antiepileptogenic Effects and MicroRNA Expression Modulation in a Rat Model of Kainate-Induced Seizures
Abstract
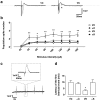
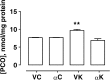
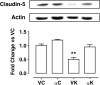
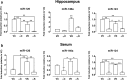
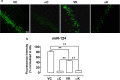
Seizure-triggered maladaptive neural plasticity and neuroinflammation occur during the latent period as a key underlying event in epilepsy chronicization. Previously, we showed that α-tocopherol (α-T) reduces hippocampal neuroglial activation and neurodegeneration in the rat model of kainic acid (KA)-induced status epilepticus (SE). These findings allowed us to postulate an antiepileptogenic potential for α-T in hippocampal excitotoxicity, in line with clinical evidence showing that α-T improves seizure control in drug-resistant patients. To explore neurobiological correlates of the α-T antiepileptogenic role, rats were injected with such vitamin during the latent period starting right after KA-induced SE, and the effects on circuitry excitability, neuroinflammation, neuronal death, and microRNA (miRNA) expression were investigated in the hippocampus. Results show that in α-T-treated epileptic rats, (1) the number of population spikes elicited by pyramidal neurons, as well as the latency to the onset of epileptiform-like network activity recover to control levels; (2) neuronal death is almost prevented; (3) down-regulation of claudin, a blood-brain barrier protein, is fully reversed; (4) neuroinflammation processes are quenched (as indicated by the decrease of TNF-α, IL-1β, GFAP, IBA-1, and increase of IL-6); (5) miR-146a, miR-124, and miR-126 expression is coherently modulated in hippocampus and serum by α-T. These findings support the potential of a timely intervention with α-T in clinical management of SE to reduce epileptogenesis, thus preventing chronic epilepsy development. In addition, we suggest that the analysis of miRNA levels in serum could provide clinicians with a tool to evaluate disease evolution and the efficacy of α-T therapy in SE.
Keywords: Epilepsy; MicroRNA; Neuroprotection; Spontaneous recurrent seizures; Vitamin E.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures


References
-
- Sutula TP. Seizure-induced plasticity and adverse long-term effects of early-life seizures. Ann Neurol. 2004;56(1):164–165. - PubMed
-
- Butler T, Li Y, Tsui W, Friedman D, Maoz A, Wang X, Harvey P, Tanzi E, Morim S, Kang Y, Mosconi L, Talos D, Kuzniecky R, Vallhabjosula S, Thesen T, Glodzik L, Ichise M, Silbersweig D, Stern E, de Leon MJ, French J. Transient and chronic seizure-induced inflammation in human focal epilepsy. Epilepsia. 2016;57(9):e191–e194. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

