Strategies to improve recruitment to randomised trials
- PMID: 29468635
- PMCID: PMC7078793
- DOI: 10.1002/14651858.MR000013.pub6
Strategies to improve recruitment to randomised trials
Abstract
Background: Recruiting participants to trials can be extremely difficult. Identifying strategies that improve trial recruitment would benefit both trialists and health research.
Objectives: To quantify the effects of strategies for improving recruitment of participants to randomised trials. A secondary objective is to assess the evidence for the effect of the research setting (e.g. primary care versus secondary care) on recruitment.
Search methods: We searched the Cochrane Methodology Review Group Specialised Register (CMR) in the Cochrane Library (July 2012, searched 11 February 2015); MEDLINE and MEDLINE In Process (OVID) (1946 to 10 February 2015); Embase (OVID) (1996 to 2015 Week 06); Science Citation Index & Social Science Citation Index (ISI) (2009 to 11 February 2015) and ERIC (EBSCO) (2009 to 11 February 2015).
Selection criteria: Randomised and quasi-randomised trials of methods to increase recruitment to randomised trials. This includes non-healthcare studies and studies recruiting to hypothetical trials. We excluded studies aiming to increase response rates to questionnaires or trial retention and those evaluating incentives and disincentives for clinicians to recruit participants.
Data collection and analysis: We extracted data on: the method evaluated; country in which the study was carried out; nature of the population; nature of the study setting; nature of the study to be recruited into; randomisation or quasi-randomisation method; and numbers and proportions in each intervention group. We used a risk difference to estimate the absolute improvement and the 95% confidence interval (CI) to describe the effect in individual trials. We assessed heterogeneity between trial results. We used GRADE to judge the certainty we had in the evidence coming from each comparison.
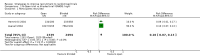
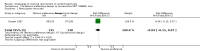
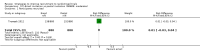
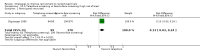
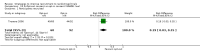
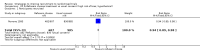
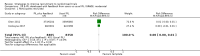
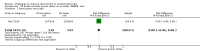
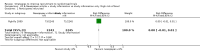
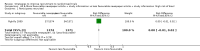
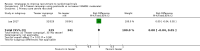
Main results: We identified 68 eligible trials (24 new to this update) with more than 74,000 participants. There were 63 studies involving interventions aimed directly at trial participants, while five evaluated interventions aimed at people recruiting participants. All studies were in health care.We found 72 comparisons, but just three are supported by high-certainty evidence according to GRADE.1. Open trials rather than blinded, placebo trials. The absolute improvement was 10% (95% CI 7% to 13%).2. Telephone reminders to people who do not respond to a postal invitation. The absolute improvement was 6% (95% CI 3% to 9%). This result applies to trials that have low underlying recruitment. We are less certain for trials that start out with moderately good recruitment (i.e. over 10%).3. Using a particular, bespoke, user-testing approach to develop participant information leaflets. This method involved spending a lot of time working with the target population for recruitment to decide on the content, format and appearance of the participant information leaflet. This made little or no difference to recruitment: absolute improvement was 1% (95% CI -1% to 3%).We had moderate-certainty evidence for eight other comparisons; our confidence was reduced for most of these because the results came from a single study. Three of the methods were changes to trial management, three were changes to how potential participants received information, one was aimed at recruiters, and the last was a test of financial incentives. All of these comparisons would benefit from other researchers replicating the evaluation. There were no evaluations in paediatric trials.We had much less confidence in the other 61 comparisons because the studies had design flaws, were single studies, had very uncertain results or were hypothetical (mock) trials rather than real ones.
Authors' conclusions: The literature on interventions to improve recruitment to trials has plenty of variety but little depth. Only 3 of 72 comparisons are supported by high-certainty evidence according to GRADE: having an open trial and using telephone reminders to non-responders to postal interventions both increase recruitment; a specialised way of developing participant information leaflets had little or no effect. The methodology research community should improve the evidence base by replicating evaluations of existing strategies, rather than developing and testing new ones.
Conflict of interest statement
Shaun Treweek and Frank Sullivan are coauthors of Treweek 2012; they were not involved in data extraction or risk of bias assessment for this study for this review. Although Shaun Treweek was not involved in Cockayne 2017, he was involved in the wider START study in which Cockayne 2017 was nested; he was not involved in data extraction or risk of bias assessment for this study for this review. Shaun Treweek was a reviewer for Jennings 2015a; Jennings 2015b; Jennings 2015c; Jennings 2015d; Jennings 2015e (all included in a single article). Shaun Treweek and Frank Sullivan declare no further conflict of interest.
Marie Pitkethly: none known.
Jonathan Cook: none known.
Cynthia Fraser: none known.
Elizabeth Mitchell: none known.
Catherine Jackson: none known.
Tyna Taskila: none known.
Heidi Gardner: none known.
Figures














































Update of
-
Strategies to improve recruitment to randomised controlled trials.Cochrane Database Syst Rev. 2010 Apr 14;(4):MR000013. doi: 10.1002/14651858.MR000013.pub5. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2018 Feb 22;2:MR000013. doi: 10.1002/14651858.MR000013.pub6. PMID: 20393971 Updated.
Comment in
-
Cochrane in CORR®: Strategies to Improve Recruitment to Randomised Trials.Clin Orthop Relat Res. 2019 Jan;477(1):22-27. doi: 10.1097/CORR.0000000000000577. Clin Orthop Relat Res. 2019. PMID: 30586062 Free PMC article. No abstract available.
References
References to studies included in this review
Abd‐Elsayed 2012 {published data only}
-
- Abd‐Elsayed AA, Sessler DI, Mendoza‐Cuartas M, Dalton JE, Said T, Meinert J, et al. A randomized controlled study to assess patients' understanding of and consenting for clinical trials using two different consent form presentations. Minerva Anestesiologica 2012;78:564‐73. - PubMed
Abhyankar 2010 {published data only}
Avenell 2004 {published data only}
-
- Avenell A, Grant AM, McGee M, McPherson G, Campbell MK, McGee MA, the RECORD Trial Management Group. The effects of an open design on trial participant recruitment, compliance and retention ‐ a randomized controlled trial comparison with a blinded, placebo‐controlled design. Clinical Trials 2004;1:490‐8. - PubMed
Bentley 2004 {published data only}
Bergenmar 2014 {published data only}
Brierley 2012 {published data only}
Chen 2011 {published and unpublished data}
-
- Chen F, Rahimi K, Haynes R, Naessens K, Taylor‐Clarke M, Murray C, et al. Investigating strategies to improve attendance at screening visits in a randomized trial. Trials 2011;12(Suppl 1):A111. [DOI: 10.1186/1745-6215-12-S1-A111] - DOI
Cockayne 2017 {published data only}
-
- Cockayne S, Fairhurst C, Adamson J, Hewitt C, Hull R, Hicks K, et al. An optimised patient information sheet did not significantly increase recruitment or retention in a falls prevention study: an embedded randomised recruitment trial. Trials 2017;18:144. [DOI: 10.1186/s13063-017-1797-7] - DOI - PMC - PubMed
Cooper 1997 {published data only}
-
- Cooper KG, Grant AM, Garratt AM. The impact of using a partially randomised patient preference design when evaluating alternative managements for heavy menstrual bleeding. Journal of Obstetrics and Gynaecology 1997;104:1367‐73. - PubMed
Coyne 2003 {published data only}
-
- Coyne CA, Xu R, Raich P, Plomer K, Dignan M, Wenzel LB, et al. Randomized, controlled trial of an easy‐to‐read informed consent statement for clinical trial participation: a study of the Eastern Cooperative Oncology Group. Journal of Clinical Oncology 2003;21(5):836‐42. - PubMed
Dear 2011 {published data only}
Diguiseppi 2006 {published data only}
-
- Diguiseppi C, Goss C, Xu S, Magid D, Graham A. Telephone screening for hazardous drinking among injured patients seen in acute care clinics: feasibility study. Alcohol & Alcoholism 2006;41(4):438‐45. - PubMed
Du 2008 {published data only}
-
- Du W, Mood D, Gadgeel S, Simon MS. An educational video to increase clinical trials enrolment among lung cancer patients. Journal of Thoracic Oncology 2008;3(1):23‐9. - PubMed
Du 2009 {published data only}
Ellis 2002 {published data only}
-
- Ellis PM, Butow PN, Tattersall MH. Informing breast cancer patients about clinical trials: a randomized clinical trial of an educational booklet. Annals of Oncology 2002;13:1414‐23. - PubMed
Fleissig 2001 {published data only}
-
- Fleissig A, Jenkins V, Fallowfield L. Results of an intervention study to improve communication about randomised clinical trials of cancer therapy. Euorpean Journal of Cancer 2001;37:322‐31. - PubMed
Ford 2004 {published data only}
-
- Ford ME, Havstad SL, Davis SD. A randomized trial of recruitment methods for older African American men in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. Clinical Trials 2004;1:343‐51. - PubMed
Foss 2016 {published and unpublished data}
Fowell 2006 {published data only}
-
- Fowell A, Johnstone R, Finlay IG, Russell D, Russell IT. Design of trials with dying patients: a feasibility study of cluster randomisation versus randomised consent. Palliative Medicine 2006;20:799‐804. - PubMed
Fracasso 2013 {published data only}
Free 2010 {published and unpublished data}
-
- Free C, Hoile E, Robertson S, Knight R. Three controlled trials of interventions to increase recruitment to a randomized controlled trial of mobile phone based smoking cessation support. Clinical Trials 2010;7:265‐73. - PubMed
Free 2011 {published data only}
Freer 2009 {published and unpublished data}
-
- Freer Y, McIntosh N, Teunisse S, Kanwaljeet JSA, Boyle EM. More information, less understanding: a randomized study on consent issues in neonatal research. Pediatrics 2009;123:1301‐5. - PubMed
Fureman 1997 {published data only}
-
- Fureman I, Meyers K, McLellan AT, Metzger D, Woody G. Evaluation of a video‐supplement to informed consent: injection drug users and preventive HIV vaccine efficacy trials. AIDS Education and Prevention 1997;9(4):330‐41. - PubMed
Graham 2007 {published data only}
-
- Graham A, Goss C, Xu S, Magid D, Diguiseppi C. Effect of using different modes to administer the AUDIT‐C on identification of hazardous drinking and acquiescence to trial participation among injured patients. Alcohol & Alcoholism 2007;42(5):423‐9. - PubMed
Halpern 2004 {published data only}
-
- Halpern SD, Karlawish JHT, Casarett, D, Berlin JA, Asch DA. Empirical assessment of whether moderate payments are undue or unjust inducements for participation in clinical trials. Annals of Internal Medicine 2004;164:801‐3. - PubMed
Hemminki 2004 {published data only}
-
- Hemminki E, Hovi SL, Veerus P, Sevon T, Tuimala R, Rahu M, et al. Blinding decreased recruitment in a prevention trial of postmenopausal hormone therapy. Journal of Clinical Epidemiology 2004;57:1237‐43. - PubMed
Hutchison 2007 {published data only}
Ives 2001 {published data only}
-
- Ives NJ, Troop M, Waters A, Davies S, Higgs C, Easterbrook PJ. Does an HIV clinical trial information booklet improve patient knowledge and understanding of HIV clinical trials?. HIV Medicine 2001;2:241‐9. - PubMed
Jacobsen 2012 {published data only}
-
- Jacobsen PB, Wells KJ, Meade CD, Quinn GP, Lee JH, Fulp WJ, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. Journal of Clinical Oncology 2012;30:2516‐21. [DOI: 10.1200/JCO.2011.39.5186] - DOI - PMC - PubMed
Jennings 2015a {published data only}
Jennings 2015b {published data only}
Jennings 2015c {published data only}
Jennings 2015d {published data only}
Jennings 2015e {published data only}
Jeste 2009 {published data only}
Karunaratne 2010 {published data only}
-
- Karunaratne A, Koremann SG, Thomas SL, Myles PS, Komeraroff PA. Improving communication when seeking informed consent: a randomised controlled study of a computer‐based method for providing information to prospective clinical trial participants. Medical Journal of Australia 2010;192:388‐92. - PubMed
Kendrick 2001 {published data only}
Kerr 2004 {published and unpublished data}
-
- Kerr CEP, Robinson EJ, Lilford RL, Edwards SLJ, Braunholtz DA, Stevens AJ. The impact of describing clinical trial treatments as new or standard. Patient Education and Counseling 2004;53:107‐13. - PubMed
Kimmick 2005 {published data only}
-
- Kimmick GG, Peterson BL, Kornblith AB, Mandelblatt J, Johnson JL, Wheeler J, et al. Improving accrual of older persons to cancer treatment trials: a randomized trial comparing an educational intervention with standard information: CALGB 360001. Journal of Clinical Oncology 2005;23(10):2201‐7. - PubMed
Larkey 2002 {published data only}
-
- Larkey LK, Staten LK, Ritenbaugh C, Hall RA, Buller DB, Bassford T, et al. Recruitment of Hispanic women to the Women's Health Initiative: the case of Embajadoras in Arizona. Controlled Clinical Trials 2002;23:289‐98. - PubMed
Lee 2017 {published data only}
Liénard 2006 {published data only}
-
- Liénard J‐L, Quinax E, Fabre‐Guillevin E, Piedbois P, Jouhaud A, Decoster G, et al. Impact of on‐site initiation visits on patient recruitment and data quality in a randomized trial of adjuvant chemotherapy for breast cancer. Clinical Trials 2006;3:486‐92. - PubMed
Litchfield 2005 {published data only}
-
- Litchfield J, Freeman J, Schou H, Elsley M, Fuller R, Chubb B. Is the future for clinical trials internet‐based? A cluster randomized clinical trial. Clinical Trials 2005;2:72‐9. - PubMed
Llewellyn‐Thomas 1995a {published data only}
-
- Llewellyn‐Thomas HA, McGreal MJ, Thiel EC. Cancer patients decision making and trial‐entry preferences: the effects of framing information about short‐term toxicity and long‐term survival. Medical Decision Making 1995;15:4‐12. - PubMed
Llewellyn‐Thomas 1995b {published data only}
-
- Llewellyn‐Thomas HA, Thiel EC, Sem FWC, Woermke DEH. Presenting clinical trial information: a comparison of methods. Patient Education and Counseling 1995;25:97‐107. - PubMed
MacQueen 2014 {published data only}
Man 2015a {published data only}
-
- Man MS, on behalf of the Healthlines Study Group, Rick J, Bower P, on behalf of the MRC‐START Group. Improving recruitment to a study of telehealth management for long‐term conditions in primary care: two embedded, randomised controlled trials of optimised patient information materials. Trials 2015;16:309. [DOI: 10.1186/s13063-015-0820-0] - DOI - PMC - PubMed
Man 2015b {published data only}
-
- Man MS, on behalf of the Healthlines Study Group, Rick J, Bower P, on behalf of the MRC‐START Group. Improving recruitment to a study of telehealth management for long‐term conditions in primary care: two embedded, randomised controlled trials of optimised patient information materials. Trials 2015;16:309. [DOI: 10.1186/s13063-015-0820-0] - DOI - PMC - PubMed
Mandelblatt 2005 {published data only}
-
- Mandelblatt J, Kaufmann E, Sheppard VB, Pomeroy J, Kavanaugh J, Canar J, et al. Breast cancer prevention in community clinics: will low‐income Latina patients participate in clinical trials?. Preventive Medicine 2005;40:611‐8. - PubMed
Miller 1999 {published data only}
-
- Miller NL, Markowitz JC, Kocsis JH, Leon AC, Brisco ST, Garno JL. Cost effectiveness of screening for clinical trials by research assistants versus senior investigators. Journal of Psychiatric Research 1999;33:81‐5. - PubMed
Monaghan 2007 {published data only}
-
- Monaghan H, Richens A, Colman S, Currie R, Girgis S, Jayne K, et al. A randomised trial of the effects of an additional communication strategy on recruitment into a large‐scale, multi‐centre trial. Contemporary Clinical Trials 2007;28:1‐5. - PubMed
Mudano 2013 {published data only}
-
- Mudano AS, Gary LC, Oliveira A, Wright MM, Curtis J, Delzell E, et al. Using tablet computers compared to interactive voice response to improve subject recruitment in osteoporosis pragmatic clinical trials: feasibility, satisfaction, and sample size. Patient Preference and Adherence 2013;7:517‐23. [DOI: 10.2147/PPA.S44551] - DOI - PMC - PubMed
Myles 1999 {published data only}
-
- Myles PS, Fletcher HE, Cairo S, Madder H, McRae R, Cooper J, et al. Randomized trial of informed consent and recruitment for clinical trials in the immediate preoperative period. Anesthesiology 1999;91:969‐78. - PubMed
Nystuen 2004 {published data only}
-
- Nystuen P, Hagen KB. Telephone reminders are effective in recruiting nonresponding patients to randomized controlled trials. Journal of Clinical Epidemiology 2004;57:773‐6. - PubMed
Paul 2011 {published data only}
-
- Paul J, Iveson T, Midgely R, Harkin A, Masterton M, Alexander L, Cassidy J. Choice of randomisation time‐point in non‐inferiority studies of reduced treatment duration: experience from the SCOT study. Trials 2011;12:A30.
Paul 2014 {published data only}
-
- Paul C, Courtney R, Sanson‐Fisher R, Carey M, Hill D, Simmons J, Rose S. A randomized controlled trial of the effectiveness of a pre‐recruitment primer letter to increase participation in a study of colorectal screening and surveillance. BMC Medical Research Methodology 2014;14:44. [DOI: 10.1186/1471-2288-14-44] - DOI - PMC - PubMed
Perrone 1995 {published data only}
-
- Perrone F, Placido S, Giusti C, Gallo C. Looking for consent in RCTs: a randomised trial with surrogate patients [La richiesta del consenso nella ricerca clinica: uno studio randomizzato in soggetti sani]. Epidemiologia e Prevenzione 1995;19:282‐90. - PubMed
Pighills 2009 {published data only}
-
- Pighills A, Torgerson DJ, Sheldon T. Publicity does not increase recruitment to falls prevention trials: the results of two quasi‐randomized trials. Journal of Clinical Epidemiology 2009;62:1332‐5. - PubMed
Simel 1991 {published data only}
-
- Simel DL, Feussner JR. A randomized controlled trial comparing quantitative informed consent formats. Journal of Clinical Epidemiology 1991;44(8):771‐7. - PubMed
Simes 1986 {published data only}
Tehranisa 2014 {published data only}
Tilley 2012 {published data only}
Treschan 2003 {published data only}
-
- Treschan TA, Scheck T, Kober A, Fleischmann E, Birkenberg B, Petschnigg B, et al. The influence of protocol pain and risk on patients' willingness to consent for clinical studies: a randomized trial. Economics, Education, and Health Systems Research 2003;96:498‐506. - PubMed
Trevena 2006 {published data only}
Treweek 2012 {published data only}
Wadland 1990 {published data only}
-
- Wadland WC, Hughes JR, Secker‐Walker RH, Bronson DL, Fenwick J. Recruitment in a primary care trial on smoking cessation. Family Medicine 1990;22:201‐4. - PubMed
Weinfurt 2008a {published data only}
Weinfurt 2008b {published data only}
Wells 2013 {published data only}
Welton 1999 {published data only}
Weston 1997 {published data only}
-
- Weston J, Hannah M, Downes J. Evaluating the benefits of a patient information video during the informed consent process. Patient Education and Counseling 1997;30:239‐45. - PubMed
Wong 2013 {published data only}
References to studies excluded from this review
Aalborg 2012 {published data only}
-
- Aalborg AE, Miller BA, Husson G, Byrnes HF, Bauman KE, Spoth RL. Implementation of adolescent family‐based substance use prevention programs in health care settings: comparisons across conditions and programs. Health Education Journal 2012;71:53‐61. [DOI: 10.1177/0017896910386209] - DOI - PMC - PubMed
Aaronson 1996 {published data only}
-
- Aaronson NK, Visser‐Pol E, Gerleen HMW, Muller MJ, Schot AC, Dam FS, et al. Telephone‐based nursing intervention improves the effectiveness of the informed consent process in cancer clinical trials. Journal of Clinical Oncology 1996;14:984‐96. - PubMed
Agoritsas 2010 {published data only}
-
- Agoritsas T, Deom M, Perneger TV. Study design attributes influenced patients' willingness to participate in clinical research: a randomized vignette‐based study. Journal of Clinical Epidemiology 2011;64:107‐15. [DOI: ] - PubMed
Alexander 2008 {published data only}
Andrew 1993 {published data only}
-
- Andrew M, Vegh P, Caco C, Kirpalani H, Jefferies A, Ohlsson A, et al. A randomized, controlled trial of platelet transfusions in thrombocytopenic premature infants. Journal of Pediatrics 2003;123:285‐91. - PubMed
Barnard 2010 {published data only}
Berman 2005 {published data only}
-
- Berman SR, Friedman EC, Callan JO, Fasiczka AL, McLean M, Hunter DM, et al. Recruitment and screening strategies for bipolar clinical trials. Bipolar Disorders 2005;7(Suppl 2):27‐117.
Brach 2013 {published data only}
-
- Brach M, Moschny A, Bücker B, Klaaßen‐Mielke R, Trampisch M, Wilm S, et al. Recruiting hard‐to‐reach subjects for exercise interventions: a multi‐centre and multi‐stage approach targeting general practitioners and their community‐dwelling and mobility‐limited patients. International Journal of Environmental Research and Public Health 2013;10:6611‐29. [DOI: 10.3390/ijerph10126611] - DOI - PMC - PubMed
Brealey 2007 {published data only}
-
- Brealey SD, Atwell C, Bryan S, Coulton S, Cox H, Cross B, et al. Using postal randomization to replace telephone randomization had no significant effect on recruitment of patients. Journal of Clinical Epidemiology 2006;60:1046‐51. - PubMed
Breland‐Noble 2012 {published data only}
Brocklehurst 2007 {published data only}
-
- Brocklehurst P, Tarnow‐Mordi W, Farrell B, Quigley M. INLET trial ‐ cluster trial of newsletters and educational supplements to centres participating in INIS trial. Oxford: National Perinatal Epidemiology Unit; 2007. Annual Report 2006.
Brown 2012 {published data only}
Burns 2008 {published data only}
-
- Burns D, Soward AC, Skelly AH, Leeman J, Carlson J. Effective recruitment and retention strategies for older members of rural minorities. Diabetes Educator 2008;34:1045‐52. - PubMed
Caldwell 2002 {published data only}
-
- Caldwell P, Craig J, Hamilton S. [Strategies for recruitment to RCTs: a systematic review of controlled trials and observational studies]. International Clinical Trials Symposium: improving health care in the new millennium, 2002 October 21‐23; Sydney. 2002:34‐5.
Calimlim 1977 {published data only}
-
- Calimlim J, Wardell WM, Lasagna L. Selection, attrition, and consent in recruitment of patients for a clinical trial. Clinical Pharmacology & Therapeutics 1977;21:100.
Carney 2014 {published data only}
-
- Carney PA, Tucker EK, Newby TA, Beer TM. Feasibility, acceptability and findings from a pilot randomized controlled intervention study on the impact of a book designed to inform patients about cancer clinical trials. Journal of Cancer Education 2014;29(1):181‐7. [DOI: 10.1007/s13187-013-0567-9] - DOI - PMC - PubMed
Celentano 1995 {published data only}
-
- Celentano DD, Beyrer C, Natpratan C, Eiumtrakul S, Sussman L, Renzullo PO, et al. Willingness to participate in AIDS vaccine trials among high‐risk populations in northern Thailand. AIDS 1995;9:1079‐83. - PubMed
Chin Feman 2008 {published data only}
Chlebowski 2010 {published data only}
Clagett 2013 {published data only}
-
- Clagett B, Nathanson KL, Ciosek SL, McDermoth M, Vaughn DJ, Mitra N, et al. Comparison of address‐based sampling and random‐digit dialing methods for recruiting young men as controls in a case‐control study of testicular cancer susceptibility. American Journal of Epidemiology 2013;178(11):1638‐47. [DOI: 10.1093/aje/kwt164] - DOI - PMC - PubMed
Cook 2010 {published data only}
-
- Cook ED, Arnold KB, Hermos JA, McCaskill‐Stevens W, Moody‐Thomas S, Probstfield JL, et al. Impact of supplemental site grants to increase African American accrual for the Selenium and Vitamin E Cancer Prevention Trial. Clinical Trials 2010;7(1):90‐9. [DOI: 10.1177/1740774509357227] - DOI - PMC - PubMed
Coronado 2012 {published data only}
Dal‐Ré 1991 {published data only}
-
- Dal‐Ré R. Clinical research with drugs: a study of the influence of information about adverse reactions on obtaining informed consent [Investigación clínica con fármacos: estudio de la influencia de la informacíon sobre reacciones adversas en la obtención del consentimiento informado]. Medicina Clínica (Barcelona) 1991;96:566‐9. - PubMed
Davis 1998 {published data only}
-
- Davis TC, Holcombe RF, Berkel HJ, Pramanik S, Divers SG. Informed consent for clinical trials: a comparative study of standard versus simplified forms. Journal of the National Cancer Institute 1998;90:668‐74. - PubMed
Donovan 2009 {published data only}
-
- Donovan J, Lane JA, Peters TJ, Brindle L, Salter E, Gillatt D, et al. Development of a complex intervention improved randomization and informed consent in a randomized controlled trial. Journal of Clinical Epidemiology 2009;62:29‐36. - PubMed
Donovan 2010 {published data only}
-
- Donovan J, Lane A, Mills N, Neal D, Hamdy F, the ProtecT Study Group. Development and application of a complex intervention to improve recruitment to randomised controlled trials. Clinical Trials 2010;7:469.
Eckardt 2011 {published data only}
-
- Eckardt JR, DeMaggio AW, Peracha O, Levonyak M, Ku N. Impact of direct physician‐to‐physician contact on accelerating oncology clinical trials accrual. Journal of Clinical Oncology 2011;29(15 Suppl).
Embi 2012 {published data only}
Enama 2012 {published data only}
-
- Enama ME, Hu Z, Gordon I, Costner P, Ledgerwood JE, Grady C, VRC 306 and 307 Consent Study Teams. Randomization to standard and concise informed consent forms: development of evidence‐based consent practices. Contemporary Clinical Trials 2012;33(5):895‐902. [DOI: 10.1016/j.cct.2012.04.005] - DOI - PMC - PubMed
Feman 2008 {published data only}
Foradori 2012 {published data only}
Gallo 1995 {published data only}
-
- Gallo C, Perrone F, Placido S, Giusti C. Informed versus randomised consent to clinical trials. Lancet 1995;346:1060‐4. - PubMed
Gillan 2009 {published data only}
Gilligan 2014 {published data only}
Gillon 2009 {published data only}
-
- Gillan MG, Gilbert FJ, Flight H, Cooper J, Wallis MG, James JJ, et al. Increasing participant recruitment into large‐scale screening trials: experience from the CADET II study. Journal of Medical Screening 2009;16:180‐5. - PubMed
Ginexi 2003 {published data only}
-
- Ginexi EM, Crosse SB, Caudill BD. Alcohol abuse prevention programming for fraternity members: are we reaching the heaviest drinkers?. 11th Annual Meeting of the Society for Research Prevention. 2003.
Gitanjali 2003 {published data only}
-
- Gitanjali B, Raveendran R, Pandian DG, Sujindra S. Recruitment of subjects for clinical trials after informed consent: does gender and educational status make a difference?. Stroke 2003;34:e109‐37. - PubMed
Goldstein 2010 {published data only}
Gomez 1998 {published data only}
-
- Gómez Arnáu JI. Preoperative information and informed consent from surgical patients. Revista Española de Anestesiologia y Reanimacion 1998;45(9):401. - PubMed
Graham 2011 {published data only}
Grubbs 2009 {published data only}
-
- Grubbs SS, Gonzalez M, Krasna M, Siegel R, Bryant D, Tschetter L, et al. Tracking clinical trial accrual strategies and barriers via a Web‐based screening tool. Journal of Clinical Oncology 2009;27(15 Suppl).
Halpern 2002 {published data only}
-
- Halpern S. Challenges to improving the impact of worksite cancer prevention programs: comparing reach, enrollment, and attrition using active versus passive recruitment strategies. Controlled Clinical Trials 2002;24:274‐88. - PubMed
Harris 2008 {published data only}
-
- Harris TJ, Carey IM, Victor CR, Adams R, Cook DG. Optimising recruitment into a study of physical activity in older people: a randomised controlled trial of different approaches. Age and Ageing 2008;37:659‐65. - PubMed
Harron 2012 {published data only}
Heiney 2010 {published data only}
Henkel 2010 {published data only}
-
- Henkel V, Mergl R, Allgaier AK, Hautzinger M, Kohnen R, Coyne JC, et al. Treatment of atypical depression: post‐hoc analysis of a randomized controlled study testing the efficacy of sertraline and cognitive behavioural therapy in mildly depressed outpatients. European Psychiatry 2010;25(8):491‐8. [DOI: 10.1016/j.eurpsy.2010.01.010] - DOI - PubMed
Hillsdon 2011 {published data only}
-
- Hillsdon M. Rates of recruitment from systematic and opportunistic methods: preliminary results from the DDELPHI study. Trials 2011;12(Suppl 1):A112.
Hoffner 2011 {published data only}
Homish 2009 {published data only}
Jaffee 2009 {published data only}
Jay 2007 {published data only}
-
- Jay F, Chantler T, Lees A, Pollard AJ. Children's participation in vaccine research: parents' views. Paediatric Nursing 2007;19:14‐8. - PubMed
Jenkins 2013 {published data only}
Ji 2008 {published data only}
-
- Ji P, DuBois DL, Flay BR, Brechling V. "Congratulations, you have been randomized into the control group!(?)": issues to consider when recruiting schools for matched‐pair randomized control trials of prevention programs. Journal of School Health 2008;78:131‐9. - PubMed
Junghans 2005 {published data only}
Juraskova 2014 {published data only}
-
- Juraskova I, Butow P, Bonner C, Bell ML, Smith AB, Seccombe M, et al. Improving decision making about clinical trial participation ‐ a randomised controlled trial of a decision aid for women considering participation in the IBIS‐II breast cancer prevention trial. British Journal of Cancer 2014;111(1):1‐7. [DOI: 10.1038/bjc.2014.144] - DOI - PMC - PubMed
Karlawish 2008 {published data only}
Keedy 2009 {published data only}
-
- Keedy VL, Horn L, Hayes A, Spencer B, Garcia G, Campbell N, et al. Enrollment of lung cancer patients on clinical trials at an NCI comprehensive cancer center. Journal of Clinical Oncology 2009;27(Suppl 15):355s.
Kelechi 2010 {published data only}
Kernan 2009 {published data only}
Kiernan 2000 {published data only}
-
- Kiernan M, Phillips K, Fair J, King AC. Using direct mail to recruit Hispanic adults into a dietary intervention: an experimental study. Annals of Behavioral Medicine 2000;22(1):89‐93. - PubMed
Kirkby 2013 {published data only}
Korde 2009 {published data only}
Kruse 2000 {published data only}
-
- Kruse AY, Kjaergard LL, Krogsgaard K, Gluud C, Mortensen EL, Gottschau A, et al. A randomized trial assessing the impact of written information on outpatients' knowledge about and attitude toward randomized clinical trials. The INFO trial group. Controlled Clinical Trials 2000;21:223‐40. - PubMed
Labrique 2011 {published data only}
Lancet 2001 {published data only}
-
- Recruitment of women to clinical trials. Lancet 2001;358:853. - PubMed
Lang 1991 {published data only}
-
- Lang JM, Buring JE, Rosner B, Cook N, Hennekens CH. Estimating the effect of the run‐in on the power of the Physicians' Health Study. Statistics in Medicine 1991;10:1585‐93. - PubMed
Larkey 2009 {published data only}
-
- Larkey LK, Gonzalez JA, Mar LE, Glantz N. Latina recruitment for cancer prevention education via Community Based Participatory Research strategies. Contemporary Clinical Trials 2009;30:47‐54. - PubMed
Leader 1978 {published data only}
-
- Leader MA, Neuwirth E. Clinical research and the noninstitutional elderly: a model for subject recruitment. Journal of the American Geriatrics Society 1978;26:27‐31. - PubMed
Lee 2011 {published data only}
-
- Lee JY, Foster HE Jr, McVary KT, Meleth S, Stavris K, Downey J, Kusek JW. Recruitment of participants to a clinical trial of botanical therapy for benign prostatic hyperplasia. Journal of Alternative and Complementary Medicine (New York) 2011;17(5):469‐72. [DOI: 10.1089/acm.2010.0300] - DOI - PMC - PubMed
Lichter 1991 {published data only}
-
- Lichter PR. Patient recruitment for clinical trials. Ophthalmology 1991;98:1489‐90. - PubMed
Lloyd‐Williams 2002 {published data only}
-
- Lloyd‐Williams F, Beaton S, Mair FS, Shiels C, Goldstein P, Hanratty B, et al. Recruiting heart failure patients to clinical trials: the experience of a randomised controlled trial in primary care. Society for Social Medicine 46th Annual Scientific Meeting. 2002.
Macias 2005 {published data only}
Marco 2008 {published data only}
-
- Marco CA. Impact of detailed informed consent on research subjects' participation: a prospective, randomized trial. Journal of Emergency Medicine 2008;34:269‐75. - PubMed
Masood 2006 {published data only}
-
- Masood J, Hafeez A, Wiseman O, Hill JT. Informed consent: are we deluding ourselves? A randomized controlled study. BJU International 2006;99:4‐5. - PubMed
May 2007 {published data only}
-
- May DE, Hallin MJ, Kratochvil CJ, Puumala SE, Smith LS, Reinecke MA, et al. Factors associated with recruitment and screening in the Treatment for Adolescents With Depression Study (TADS). Journal of the American Academy of Child and Adolescent Psychiatry 2007;46:801‐10. - PubMed
McGuire 2011 {published data only}
Menoyo 2006 {published data only}
-
- Menoyo E, Perez M, Torre R, Farré M. Telephone screening to improve recruitment of healthy volunteers in neuropsychopharmacology clinical trials: role of nurses. Proceedings of the 20th Congress of the Spanish Clinical Pharmacology Society. 2006.
Monane 1991 {published data only}
-
- Monane M, et al. The randomized controlled trial in the long‐term care setting ‐ recruitment and enrollment. Journal of the American Geriatrics Society 1991;39:A51.
Murphy 2011 {published data only}
O'Lonergan 2011 {published data only}
Olver 2009 {published data only}
-
- Olver IN, Whitford HS, Denson LA, Peterson MJ, Olver SI. Improving informed consent to chemotherapy: a randomized controlled trial of written information versus an interactive multimedia CD‐ROM. Patient Education and Counseling 2009;74:197‐204. - PubMed
Paskett 2002 {published data only}
-
- Paskett ED, Cooper MR, Stark N, Ricketts TC, Tropman S, Hatzell T, et al. Clinical trial enrollment of rural patients with cancer. Cancer Practice 2002;10:28‐35. - PubMed
Perri 2006 {published data only}
-
- Perri R, Wollin S, Drolet N, Mai S, Awad M, Feine J. Monitoring recruitment success and cost in a randomized clinical trial. European Journal of Prosthodontics & Restorative Dentistry 2006;14:126‐30. - PubMed
Porucznik 2010 {published data only}
-
- Porucznik CA, Schliep KC, Stanford JB. Participant recruitment in a virtually interconnected world: comparative effectiveness and considerations of bias. American Journal of Epidemiology 2010;171(Suppl):s147.
Quinaux 2003 {published data only}
-
- Quinaux E, Liénard JL, Slimani Z, Jouhaud A, Piedbois P, Buyse M. Impact of monitoring visits on patient recruitment and data quality: case study of a phase IV trial in oncology. Controlled Clinical Trials 2003;24:99S.
Rogers 1998 {published data only}
-
- Rogers CG, Tyson JE, Kennedy KA, Broyles RS, Hickman JF. Conventional consent with opting in versus simplified consent with opting out: an exploratory trial for studies that do not increase patient risk. Journal of Pediatrics 1998;132:606‐11. - PubMed
Rowbotham 2013 {published data only}
Ruffin 2011 {published data only}
Santoyo‐Olsson 2011 {published data only}
Saul 2002 {published data only}
-
- Saul H. Influences on recruitment in clinical trials. European Journal of Cancer 2002;38:2334.
Scholes 2007 {published data only}
-
- Scholes D, Heidrich FE, Yarbro P, Lindenbaum JE, Marrazzo JM. Population‐based outreach for Chlamydia screening in men: results from a randomized trial. Sexually Transmitted Diseases 2007;34:837‐9. - PubMed
Schrott 1982 {published data only}
-
- Schrott HG, Merideth N. Recruitment by screening entire communities. The Iowa Lipid Research Clinic Experience. Circulation 1982;66(6 Pt 2):IV23‐6. [PUBMED: 7127714] - PubMed
Schroy 2009 {published data only}
Sherman 2009 {published data only}
Swain 2011 {published data only}
Tenorio 2014 {published data only}
-
- Tenorio SL, O'Donnell CI, Hernandez J, Rozjabek HM, Lynch D, Marcus PM. Culturally sensitive approaches to recruitment and retention of Hispanics in the national lung screening trial. Journal of Immigrant and Minority Health/Center for Minority Public Health 2014;16(4):761‐4. [DOI: 10.1007/s10903-013-9862-0] - DOI - PubMed
Ubel 1997 {published data only}
-
- Ubel PA, Merz JF, Shea J, Asch DA. How preliminary data affect people's stated willingness to enter a hypothetical randomized controlled trial. Journal of Investigative Medicine 1997;45:561‐6. - PubMed
Unger 2006 {published data only}
-
- Unger JM, Coltman CA Jr, Crowley JJ, Hutchins LF, Martino S, Livingston RB, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. Journal of Clinical Oncology 2006;24:141‐4. - PubMed
Unger 2010 {published data only}
-
- Unger S, Wylie L, Fallah S, Heinrich L, O'Brien K. Motivated by money? The impact of financial incentive for the research team on study recruitment. IRB: Ethics & Human Research 2010;32(1):16‐9. [PUBMED: 20184220] - PubMed
Vaidya 2010 {published data only}
-
- Vaidya VS. Pragmatism in the TARGIT trial encouraged wider participation of centres yet yielded an unexpected homogeneous patient profile. EJC Supplements 2010;8(3):131.
Wang 2014 {published data only}
Woodford 2011 {published data only}
Wragg 2000 {published data only}
-
- Wragg JA, Robinson EJ, Lilford RJ. Information presentation and decisions to enter clinical trials: a hypothetical trial of hormone replacement therapy. Social Science and Medicine 2000;51:453‐62. - PubMed
Yates 2009 {published data only}
-
- Yates BC, Dodendorf D, Lane J, LaFramboise L, Pozehl B, Duncan K, et al. Testing an alternate informed consent process. Nursing Research 2009;58:135‐9. - PubMed
References to studies awaiting assessment
Cramer 1993 {published data only}
-
- Cramer JA. Patient recruitment and compliance issues in clinical trials. Epilepsy Research. Supplement 1993;10:211‐22. - PubMed
Glen 1980 {published data only}
-
- Glen VH. Patient involvement utilizing video cassettes. Ontario Dentist 1980;57:20‐1. - PubMed
Greenlee 2003 {published data only}
-
- Greenlee H, Gonzalez AJ, Lampe JW. Recruitment feasibility for a pilot randomized controlled trial on the effect of naturopathic therapies on estrogen metabolism. Cancer Epidemiology Biomarkers & Prevention 2003;12:1302s.
Additional references
Brueton 2013
Caldwell 2010
Charlson 1984
Clarke 2015
-
- Clarke M, Savage G, Maguire L, McAneney H. The SWAT (study within a trial) programme; embedding trials to improve the methodological design and conduct of future research. Trials 2015;16(Suppl 2):P209. [DOI: 10.1186/1745-6215-16-S2-P209] - DOI
Edwards 2009
Farrell 2017
-
- Farrell B. Status of our study. Email to S Treweek 6/4/2017.
Foy 2003
-
- Foy R, Parry J, Duggan A, Delaney B, Wilson S, Lewin‐van den Broek NTh, et al. How evidence‐based are recruitment strategies for randomized controlled trials in primary care? Experience from seven studies. Family Practice 2003;20:83‐92. - PubMed
Guyatt 2008
-
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI: 10.1136/bmj.39489.470347.AD] - DOI - PMC - PubMed
Haidich 2001
-
- Haidich AB, Ioannidis JPA. Patterns of patient enrolment in randomized controlled trials. Journal of Clinical Epidemiology 2001;54:877‐83. - PubMed
Haynes 2016
-
- Haynes R. Randomisation used on our study. Email to S Treweek 23/11/2016.
Higgins 2003
Kitterman 2011
Madurasinghe 2016
McDonald 2006
Paul 2016
-
- Paul J. Ramdomisation used in our study. Email to S Treweek 21/12/2016.
Prescott 1999
-
- Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al. Factors that limit the quality,number and progress of randomised controlled trials. Health Technology Assessment 1999;3(20):1‐143. - PubMed
Preston 2016
Rendell 2007
Sully 2013
Walters 2017
-
- Walters SJ, Bonacho dos Anjos Henriques‐Cadby I, Bortolami O, Flight L, Hind D, Jacques RM, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open 2017;7:e015276. [10.1136/bmjopen‐2016‐ 015276] - PMC - PubMed
References to other published versions of this review
Mapstone 2007
Treweek 2010
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

