Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis
- PMID: 29469650
- PMCID: PMC6219642
- DOI: 10.1080/19490976.2018.1441664
Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis
Abstract
Background: Conditions of excess androgen in women, such as polycystic ovary syndrome (PCOS), often exhibit intergenerational transmission. One way in which the risk for PCOS may be increased in daughters of affected women is through exposure to elevated androgens in utero. Hyperandrogenemic conditions have serious health consequences, including increased risk for hypertension and cardiovascular disease. Recently, gut dysbiosis has been found to induce hypertension in rats, such that blood pressure can be normalized through fecal microbial transplant. Therefore, we hypothesized that the hypertension seen in PCOS has early origins in gut dysbiosis caused by in utero exposure to excess androgen. We investigated this hypothesis with a model of prenatal androgen (PNA) exposure and maternal hyperandrogenemia by single-injection of testosterone cypionate or sesame oil vehicle (VEH) to pregnant dams in late gestation. We then completed a gut microbiota and cardiometabolic profile of the adult female offspring.
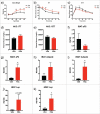
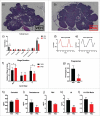
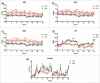
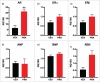
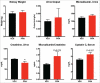
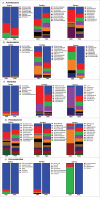
Results: The metabolic assessment revealed that adult PNA rats had increased body weight and increased mRNA expression of adipokines: adipocyte binding protein 2, adiponectin, and leptin in inguinal white adipose tissue. Radiotelemetry analysis revealed hypertension with decreased heart rate in PNA animals. The fecal microbiota profile of PNA animals contained higher relative abundance of bacteria associated with steroid hormone synthesis, Nocardiaceae and Clostridiaceae, and lower abundance of Akkermansia, Bacteroides, Lactobacillus, Clostridium. The PNA animals also had an increased relative abundance of bacteria associated with biosynthesis and elongation of unsaturated short chain fatty acids (SCFAs).
Conclusions: We found that prenatal exposure to excess androgen negatively impacted cardiovascular function by increasing systolic and diastolic blood pressure and decreasing heart rate. Prenatal androgen was also associated with gut microbial dysbiosis and altered abundance of bacteria involved in metabolite production of short chain fatty acids. These results suggest that early-life exposure to hyperandrogenemia in daughters of women with PCOS may lead to long-term alterations in gut microbiota and cardiometabolic function.
Keywords: Microbiota; PCOS; androgens; blood pressure; cardiometabolic; cardiovascular disease; cystatin C; hyperandrogenemia; hypertension; kidney; testosterone.
Figures

References
-
- Perez-Sepulveda A, Monteiro LJ, Dobierzewska A, España-Perrot PP, Venegas-Araneda P, Guzmán-Rojas AM, González Mí I, Palominos-Rivera M, Irarrazabal CE, Figueroa-Diesel H, et al.. Placental Aromatase Is Deficient in Placental Ischemia and Preeclampsia. PLoS One. 2015;10. doi: 10.1371/journal.pone.0139682. - DOI - PMC - PubMed
-
- Khattab A, Yuen T, Sun L, Yau M, Barhan A, Zaidi M, Lo YM, New MI. Noninvasive Prenatal Diagnosis of Congenital Adrenal Hyperplasia. Endocr Dev. 2016;30:37–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
