External Quality Assessment for Zika Virus Molecular Diagnostic Testing, Brazil
- PMID: 29470164
- PMCID: PMC5938781
- DOI: 10.3201/eid2405.171747
External Quality Assessment for Zika Virus Molecular Diagnostic Testing, Brazil
Abstract
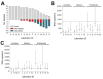
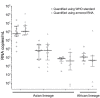
We conducted an external quality assessment of Zika virus molecular diagnostic tests in Brazil using a new Zika virus standard. Of 15 laboratories, 73% showed limited sensitivity and specificity. Viral load estimates varied significantly. Continuous quality assurance is needed to adequately estimate risk for Zika virus-associated disease and determine patient care.
Keywords: Americas; Brazil; Zika virus; diagnostics; real-time RT-PCR; surveillance; vector-borne infections; viruses.
Figures
References
-
- de Araújo TVB, Rodrigues LC, de Alencar Ximenes RA, de Barros Miranda-Filho D, Montarroyos UR, de Melo APL, et al. ; investigators from the Microcephaly Epidemic Research Group; Brazilian Ministry of Health; Pan American Health Organization; Instituto de Medicina Integral Professor Fernando Figueira; State Health Department of Pernambuco. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis. 2016;16:1356–63. 10.1016/S1473-3099(16)30318-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

