Oleanolic Acid Exerts Osteoprotective Effects and Modulates Vitamin D Metabolism
- PMID: 29470404
- PMCID: PMC5852823
- DOI: 10.3390/nu10020247
Oleanolic Acid Exerts Osteoprotective Effects and Modulates Vitamin D Metabolism
Abstract
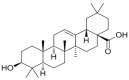
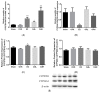
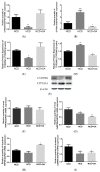
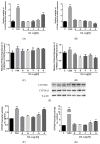
Oleanolic acid (OA) is a triterpenoid with reported bone anti-resorption activities. The present study aimed to characterize its bone protective effects in vivo and to study its effects on vitamin D metabolism, both in vivo and in vitro. OA significantly increased bone mineral density, improved micro-architectural properties, reduced urinary Ca excretion, increased 1,25(OH)₂D₃ and renal CYP27B1 mRNA expression in mature C57BL/6 ovariectomised (OVX) mice. OA also improved bone properties, Ca balance, and exerted modulatory effects on renal CYP27B1 and CYP24A1 expressions in aged normal female Sprague-Dawley rats. In addition, OA significantly increased renal CYP27B1 mRNA and promoter activity, and suppressed CYP24A1 mRNA and protein expressions in human proximal tubule HKC-8 cells. OA exerted bone protective effects in mature OVX mice and aged female rats. This action on bone might be, at least in part, associated with its effects on Ca and vitamin D metabolism. The present findings suggest that OA is a potential drug candidate for the management of postmenopausal osteoporosis.
Keywords: aging; calcium; oleanolic acid; osteoporosis; ovariectomised; vitamin D.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Ethanol Extract of Fructus ligustri lucidi Increased Circulating 1,25(OH)2D3 Levels, but Did Not Improve Calcium Balance in Mature Ovariectomized Rats.Am J Chin Med. 2016;44(6):1237-1253. doi: 10.1142/S0192415X16500695. Epub 2016 Sep 15. Am J Chin Med. 2016. PMID: 27627920
-
Generation of 1,25-dihydroxyvitamin D3 in Cyp27b1 knockout mice by treatment with 25-hydroxyvitamin D3 rescued their rachitic phenotypes.J Steroid Biochem Mol Biol. 2019 Jan;185:71-79. doi: 10.1016/j.jsbmb.2018.07.012. Epub 2018 Jul 18. J Steroid Biochem Mol Biol. 2019. PMID: 30031146
-
Total flavonoid fraction of the Herba epimedii extract suppresses urinary calcium excretion and improves bone properties in ovariectomised mice.Br J Nutr. 2011 Jan;105(2):180-9. doi: 10.1017/S0007114510003247. Epub 2010 Sep 6. Br J Nutr. 2011. PMID: 20815976
-
[Frontiers in vitamin D; basic research and clinical application. Eldecalcitol: the effect on bones and calcium metabolism].Clin Calcium. 2011 Nov;21(11):103-10. Clin Calcium. 2011. PMID: 22040826 Review. Japanese.
-
Analysis of vitamin D metabolism gene expression in human bone: evidence for autocrine control of bone remodelling.J Steroid Biochem Mol Biol. 2014 Oct;144 Pt A:110-3. doi: 10.1016/j.jsbmb.2013.09.016. Epub 2013 Oct 10. J Steroid Biochem Mol Biol. 2014. PMID: 24120913 Review.
Cited by
-
Icariin, an Up-and-Coming Bioactive Compound Against Neurological Diseases: Network Pharmacology-Based Study and Literature Review.Drug Des Devel Ther. 2021 Aug 20;15:3619-3641. doi: 10.2147/DDDT.S310686. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34447243 Free PMC article. Review.
-
Evolving Roles of Natural Terpenoids From Traditional Chinese Medicine in the Treatment of Osteoporosis.Front Endocrinol (Lausanne). 2022 May 16;13:901545. doi: 10.3389/fendo.2022.901545. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35651977 Free PMC article. Review.
-
Oleanolic Acid and Ursolic Acid Improve Bone Properties and Calcium Balance and Modulate Vitamin D Metabolism in Aged Female Rats.Front Pharmacol. 2018 Dec 4;9:1435. doi: 10.3389/fphar.2018.01435. eCollection 2018. Front Pharmacol. 2018. PMID: 30564129 Free PMC article.
-
Human breastmilk-derived Bifidobacterium longum subsp. infantis CCFM1269 regulates bone formation by the GH/IGF axis through PI3K/AKT pathway.Gut Microbes. 2024 Jan-Dec;16(1):2290344. doi: 10.1080/19490976.2023.2290344. Epub 2023 Dec 20. Gut Microbes. 2024. PMID: 38116652 Free PMC article.
-
Terpenoids as Potential Geroprotectors.Antioxidants (Basel). 2020 Jun 17;9(6):529. doi: 10.3390/antiox9060529. Antioxidants (Basel). 2020. PMID: 32560451 Free PMC article. Review.
References
-
- Halloran B.P., Portale A.A. Vitamin D metabolism and aging. In: Feldman D., Pike J.W., Glorieux F., editors. Vitamin D. 2nd ed. Elsevier Academic Press; San Diego, CA, USA: 2005. pp. 823–838.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

