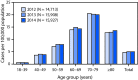
Prevalence of Amyotrophic Lateral Sclerosis - United States, 2014
- PMID: 29470458
- PMCID: PMC5858037
- DOI: 10.15585/mmwr.mm6707a3
Prevalence of Amyotrophic Lateral Sclerosis - United States, 2014
Abstract
Amyotrophic lateral sclerosis (ALS), commonly known as Lou Gehrig's disease, is a progressive and fatal neuromuscular disease; the majority of ALS patients die within 2-5 years of receiving a diagnosis (1). Familial ALS, a hereditary form of the disease, accounts for 5%-10% of cases, whereas the remaining sporadic cases have no clearly defined etiology (1). ALS affects persons of all races and ethnicities; however, whites, males, non-Hispanics, persons aged >60 years, and those with a family history of ALS are more likely to develop the disease (1-3). No cure for ALS has yet been identified, and the lack of proven and effective therapeutic interventions is an ongoing challenge. Current treatments available do not cure ALS but have been shown to slow disease progression. Until recently, only one drug (riluzole) was approved to treat ALS; however, in 2017, the Food and Drug Administration approved a second drug, edaravone (4).
Figures
References
-
- Mitsumoto HCD, Pioro EP. Amyotrophic lateral sclerosis. Philadelphia, PA: F.A. Davis Company; 1998.
-
- Mehta P, Antao V, Kaye W, et al. Prevalence of amyotrophic lateral sclerosis—United States, 2010–2011. MMWR Suppl 2014;63(No. SS-7). - PubMed
-
- Mehta P, Kaye W, Bryan L, et al. Prevalence of amyotrophic lateral sclerosis—United States, 2012–2013. MMWR Suppl 2016;65(No. SS-7). - PubMed
-
- Food and Drug Administration. FDA approves drug to treat ALS [press release]. Washington, DC: Food and Drug Administration; 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm557102.htm
-
- ALS registry act of 2008, Pub. L. 110-373, 122 Stat 4047 (October 8, 2008).
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous