Glutathionylation of dengue and Zika NS5 proteins affects guanylyltransferase and RNA dependent RNA polymerase activities
- PMID: 29470500
- PMCID: PMC5823458
- DOI: 10.1371/journal.pone.0193133
Glutathionylation of dengue and Zika NS5 proteins affects guanylyltransferase and RNA dependent RNA polymerase activities
Abstract
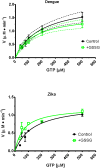
It has been estimated for dengue infection that the global population at risk is 3.5 billion people, which makes dengue an important public health problem. The causative agents of dengue are dengue viruses. For dengue virus replication, the dengue virus NS5 protein is of special importance as it has several enzyme activities important for viral replication. Previous reports of phosphorylation and SUMOylation of dengue NS5 have shown these protein modifications have important consequences for NS5 functions. In this report we identify glutathionylation, another reversible post translation modification that impacts on NS5 enzyme activity. Using dengue virus infected cells we employed specific antibodies and mass spectrometry to identify 3 cysteine residues of NS5 protein as being glutathionylated. Glutathionylation is a post translational protein modification where glutathione is covalently attached to a cysteine residue. We showed glutathionylation occurs on 3 conserved cysteine residues of dengue NS5. Then we generated two flavivirus recombinant full length proteins, dengue NS5 and Zika NS5, to characterize two of the NS5 enzyme activities, namely, guanylyltransferase and RNA-dependent RNA polymerase activities. We show glutathionylation of dengue and Zika NS5 affects enzyme activities of the two flavivirus proteins. The data suggests that glutathionylation is a general feature of the flavivirus NS5 protein and the modification has the potential to modulate several of the NS5 enzyme functions.
Conflict of interest statement
Figures





Similar articles
-
Effects of Glutathionylation on Guanylyltransferase Activity of NS5 N-terminal Capping Domain from Dengue, Japanese Encephalitis, and Zika Viruses.Protein Pept Lett. 2023;30(5):439-447. doi: 10.2174/0929866530666230418101606. Protein Pept Lett. 2023. PMID: 37076471
-
Substrate selectivity of Dengue and Zika virus NS5 polymerase towards 2'-modified nucleotide analogues.Antiviral Res. 2017 Apr;140:25-36. doi: 10.1016/j.antiviral.2016.12.021. Epub 2016 Dec 30. Antiviral Res. 2017. PMID: 28041959
-
NS5 from Dengue Virus Serotype 2 Can Adopt a Conformation Analogous to That of Its Zika Virus and Japanese Encephalitis Virus Homologues.J Virol. 2019 Dec 12;94(1):e01294-19. doi: 10.1128/JVI.01294-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597763 Free PMC article.
-
The Transactions of NS3 and NS5 in Flaviviral RNA Replication.Adv Exp Med Biol. 2018;1062:147-163. doi: 10.1007/978-981-10-8727-1_11. Adv Exp Med Biol. 2018. PMID: 29845531 Review.
-
The dengue virus NS5 protein as a target for drug discovery.Antiviral Res. 2015 Jul;119:57-67. doi: 10.1016/j.antiviral.2015.04.010. Epub 2015 Apr 24. Antiviral Res. 2015. PMID: 25912817 Review.
Cited by
-
A regulatory role for the redox status of the pepino mosaic virus coat protein.PLoS Pathog. 2023 Oct 18;19(10):e1011732. doi: 10.1371/journal.ppat.1011732. eCollection 2023 Oct. PLoS Pathog. 2023. PMID: 37851701 Free PMC article.
-
Regulation of Retroviral and SARS-CoV-2 Protease Dimerization and Activity through Reversible Oxidation.Antioxidants (Basel). 2022 Oct 18;11(10):2054. doi: 10.3390/antiox11102054. Antioxidants (Basel). 2022. PMID: 36290777 Free PMC article. Review.
-
Hepatitis C Virus RNA-Dependent RNA Polymerase Is Regulated by Cysteine S-Glutathionylation.Oxid Med Cell Longev. 2019 Sep 3;2019:3196140. doi: 10.1155/2019/3196140. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31687077 Free PMC article.
-
Role of Glutathionylation in Infection and Inflammation.Nutrients. 2019 Aug 20;11(8):1952. doi: 10.3390/nu11081952. Nutrients. 2019. PMID: 31434242 Free PMC article. Review.
-
Peptide-Based Subunit Vaccine Design of T- and B-Cells Multi-Epitopes against Zika Virus Using Immunoinformatics Approaches.Microorganisms. 2019 Jul 31;7(8):226. doi: 10.3390/microorganisms7080226. Microorganisms. 2019. PMID: 31370224 Free PMC article.
References
-
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. (2013) The global distribution and burden of dengue. Nature 496:504–7 doi: 10.1038/nature12060 - DOI - PMC - PubMed
-
- Weaver SC. (2005) Host range, amplification and arboviral disease emergence. Arch Virol Suppl:33–44 - PubMed
-
- Kraemer MU, Sinka ME, Duda KA, Mylne A, Shearer FM, Brady OJ, et al. (2015) The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci Data 2:150035 doi: 10.1038/sdata.2015.35 - DOI - PMC - PubMed
-
- Benedict MQ, Levine RS, Hawley WA, Lounibos LP. (2007) Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis 7:76–85 doi: 10.1089/vbz.2006.0562 - DOI - PMC - PubMed
-
- Atasheva S, Krendelchtchikova V, Liopo A, Frolova E, Frolov I. (2010) Interplay of acute and persistent infections caused by Venezuelan equine encephalitis virus encoding mutated capsid protein. J Virol 84:10004–15 doi: 10.1128/JVI.01151-10 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

