HIC1 links retinoic acid signalling to group 3 innate lymphoid cell-dependent regulation of intestinal immunity and homeostasis
- PMID: 29470558
- PMCID: PMC5823476
- DOI: 10.1371/journal.ppat.1006869
HIC1 links retinoic acid signalling to group 3 innate lymphoid cell-dependent regulation of intestinal immunity and homeostasis
Abstract
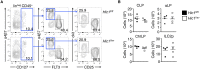
The intestinal immune system must be able to respond to a wide variety of infectious organisms while maintaining tolerance to non-pathogenic microbes and food antigens. The Vitamin A metabolite all-trans-retinoic acid (atRA) has been implicated in the regulation of this balance, partially by regulating innate lymphoid cell (ILC) responses in the intestine. However, the molecular mechanisms of atRA-dependent intestinal immunity and homeostasis remain elusive. Here we define a role for the transcriptional repressor Hypermethylated in cancer 1 (HIC1, ZBTB29) in the regulation of ILC responses in the intestine. Intestinal ILCs express HIC1 in a vitamin A-dependent manner. In the absence of HIC1, group 3 ILCs (ILC3s) that produce IL-22 are lost, resulting in increased susceptibility to infection with the bacterial pathogen Citrobacter rodentium. Thus, atRA-dependent expression of HIC1 in ILC3s regulates intestinal homeostasis and protective immunity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song S-Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21: 527–38. doi: 10.1016/j.immuni.2004.08.011 - DOI - PubMed
-
- Kim MH, Taparowsky EJ, Kim CH. Retinoic Acid Differentially Regulates the Migration of Innate Lymphoid Cell Subsets to the Gut. Immunity. 2015;43: 107–119. doi: 10.1016/j.immuni.2015.06.009 - DOI - PMC - PubMed
-
- Mora JR, von Andrian UH. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin Immunol. 2009;21: 28–35. doi: 10.1016/j.smim.2008.08.002 - DOI - PMC - PubMed
-
- Sun C-M, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204: 1775–85. doi: 10.1084/jem.20070602 - DOI - PMC - PubMed
-
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317: 256–60. doi: 10.1126/science.1145697 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

