Defining the Most Appropriate Primary End Point in Phase 2 Trials of Immune Checkpoint Inhibitors for Advanced Solid Cancers: A Systematic Review and Meta-analysis
- PMID: 29470579
- PMCID: PMC5885200
- DOI: 10.1001/jamaoncol.2017.5236
Defining the Most Appropriate Primary End Point in Phase 2 Trials of Immune Checkpoint Inhibitors for Advanced Solid Cancers: A Systematic Review and Meta-analysis
Abstract
Importance: Checkpoint inhibitors have a unique mechanism of action that differs from chemotherapy or targeted therapies. The validity of objective response rate (ORR) as a surrogate for progression-free survival (PFS) and overall survival (OS) in checkpoint-inhibitor trials is uncertain.
Objective: To determine the types of primary end points used in phase 2 checkpoint-inhibitor trials, and to assess the strength of associations for ORR with PFS and OS.
Data sources: Trials listed in electronic databases from 2000 to 2017 (PREMEDLINE, MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials).
Study selection: Advanced solid cancers in phase 2 and phase 3 trials.
Data extraction and synthesis: Correlations between ORR odds ratios and hazard ratios (HRs) for PFS and OS were examined for randomized comparisons. Within checkpoint-inhibitor treatment arms, correlations for ORR with 6-month PFS and 12-month OS rates were examined. All analyses were weighted by trial size. Multivariable models to predict 6-month PFS and 12-month OS rates from ORR were developed and their performance validated in an independent sample of trials.
Main outcomes and measures: Correlation coefficient (r) of ORR with PFS and OS.
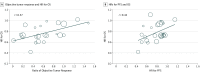
Results: Of 87 phase 2 trials identified, ORR was the most common (52 [60%]) primary end point. Twenty randomized clinical trials with 25 treatment comparisons were identified. Checkpoint-inhibitor therapy was associated with pooled ORR of 24% (95% CI, 18%-31%). For randomized comparisons, r between ORR odds ratio and PFS HR was 0.63 (95% CI, 0.35-0.89), ORR odds ratio and OS HR was 0.57 (95% CI, 0.23-0.89), and between PFS HR and OS HR was 0.42 (95% CI, 0.04-0.81). Within the checkpoint-inhibitor arms, r correlation coefficients between ORR with 6-month PFS, ORR with 12-month OS, and 6-month PFS with 12-month OS were 0.37 (95% CI, -0.06 to 0.95), 0.08 (95% CI, -0.17 to 0.70), and 0.74 (95% CI, 0.57-0.92), respectively. In validation, when 6-month PFS was used to predict 12-month OS, there was a good calibration between actual and predicted 12-month OS. When ORR was used to predict 6-month PFS and 12-month OS rates, respectively, the actual vs predicted rates calibrated poorly.
Conclusions and relevance: In checkpoint-inhibitor trials, ORR correlated poorly with OS. For future phase 2 studies, 6-month PFS rate is recommended as an end point.
Conflict of interest statement
Figures



Comment in
-
Surrogate study endpoints in the era of cancer immunotherapy.Ann Transl Med. 2018 Nov;6(Suppl 1):S27. doi: 10.21037/atm.2018.09.31. Ann Transl Med. 2018. PMID: 30613602 Free PMC article. No abstract available.
-
When Can Intermediate Outcomes Be Used as Surrogate Outcomes?JAMA. 2020 Mar 24;323(12):1184-1185. doi: 10.1001/jama.2020.1176. JAMA. 2020. PMID: 32105291 No abstract available.
References
-
- Johnson JR, Ning Y-M, Farrell A, Justice R, Keegan P, Pazdur R. Accelerated approval of oncology products: the Food and Drug Administration experience. J Natl Cancer Inst. 2011;103(8):636-644. - PubMed
-
- Therasse P, Arbuck SG, Eisenhauer EA, et al. . New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205-216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

