Mucosa-associated invariant T cells in malignancies: a faithful friend or formidable foe?
- PMID: 29470597
- PMCID: PMC11028145
- DOI: 10.1007/s00262-018-2132-1
Mucosa-associated invariant T cells in malignancies: a faithful friend or formidable foe?
Abstract
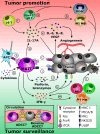
Mucosa-associated invariant T (MAIT) cells are a subset of innate-like T lymphocytes known for their ability to respond to MHC-related protein 1 (MR1)-restricted stimuli and select cytokine signals. They are abundant in humans and especially enriched in mucosal layers, common sites of neoplastic transformation. MAIT cells have been found within primary and metastatic tumors. However, whether they promote malignancy or contribute to anticancer immunity is unclear. On the one hand, MAIT cells produce IL-17A in certain locations and under certain circumstances, which could in turn facilitate neoangiogenesis, intratumoral accumulation of immunosuppressive cell populations, and cancer progression. On the other hand, they can express a potent arsenal of cytotoxic effector molecules, NKG2D and IFN-γ, all of which have established roles in cancer immune surveillance. In this review, we highlight MAIT cells' characteristics as they might pertain to cancer initiation, progression, or control. We discuss recent findings, including our own, that link MAIT cells to cancer, with a focus on colorectal carcinoma, as well as some of the outstanding questions in this active area of research. Finally, we provide a hypothetical picture in which MAIT cells constitute attractive targets in cancer immunotherapy.
Keywords: CITIM 2017; Cancer; IFN-γ; IL-17; MAIT cells; Tumor-infiltrating leukocytes.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178(1):1–16. doi: 10.1084/jem.178.1.1. - DOI - PMC - PubMed
-
- Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, Bendelac A, Bonneville M, Lantz O. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189(12):1907–1921. doi: 10.1084/jem.189.12.1907. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

