Branching the Tel2 pathway for exact fit on phosphatidylinositol 3-kinase-related kinases
- PMID: 29470645
- PMCID: PMC6842304
- DOI: 10.1007/s00294-018-0817-9
Branching the Tel2 pathway for exact fit on phosphatidylinositol 3-kinase-related kinases
Abstract
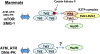
Phosphatidylinositol 3-kinase-related kinases (PIKKs), are structurally related to phosphatidylinositol 3-kinase (lipid kinase), but possess protein kinase activities. PIKKs include ATM, ATR, DNA-PK, mTOR and SMG1, key regulators of cell proliferation and genome maintenance. TRRAP, which is devoid of protein kinase activity, is the sixth member of the PIKK family. PIKK family members are gigantic proteins in the range of 300-500 kDa. It has become apparent in the last decade that the stability or maturation of the PIKK family members depends on a molecular chaperone called the Tel2-Tti1-Tti2 (TTT) complex. Several lines of evidence have established a model in which TTT connects to the Hsp90 chaperone through the Rvb1-Rvb2-Tah1-Pih1 (R2TP) complex in mammalian and yeast cells. However, recent studies of yeast cells indicate that TTT is able to form different complexes. These observations raise a possibility that several different mechanisms regulate TTT-mediated protein stability of PIKKs.
Keywords: Asa1; Casein kinase; Cdc37; Mec1; Protein folding; Tel1.
Figures


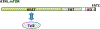


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

