Effectiveness of Practices to Support Appropriate Laboratory Test Utilization: A Laboratory Medicine Best Practices Systematic Review and Meta-Analysis
- PMID: 29471324
- PMCID: PMC6016712
- DOI: 10.1093/ajcp/aqx147
Effectiveness of Practices to Support Appropriate Laboratory Test Utilization: A Laboratory Medicine Best Practices Systematic Review and Meta-Analysis
Abstract
Objectives: To evaluate the effectiveness of practices used to support appropriate clinical laboratory test utilization.
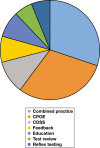
Methods: This review followed the Centers for Disease Control and Prevention (CDC) Laboratory Medicine Best Practices A6 cycle method. Eligible studies assessed one of the following practices for effect on outcomes relating to over- or underutilization: computerized provider order entry (CPOE), clinical decision support systems/tools (CDSS/CDST), education, feedback, test review, reflex testing, laboratory test utilization (LTU) teams, and any combination of these practices. Eligible outcomes included intermediate, systems outcomes (eg, number of tests ordered/performed and cost of tests), as well as patient-related outcomes (eg, length of hospital stay, readmission rates, morbidity, and mortality).
Results: Eighty-three studies met inclusion criteria. Fifty-one of these studies could be meta-analyzed. Strength of evidence ratings for each practice ranged from high to insufficient.
Conclusion: Practice recommendations are made for CPOE (specifically, modifications to existing CPOE), reflex testing, and combined practices. No recommendation for or against could be made for CDSS/CDST, education, feedback, test review, and LTU. Findings from this review serve to inform guidance for future studies.
Figures








References
-
- Balogh E, Miller BT, Ball J; Institute of Medicine (U.S.) Committee on Diagnostic Error in Health Care: Improving Diagnosis in Health Care. Washington, DC: The National Academies Press; 2015. xxvii, 444. - PubMed
-
- Hallworth MJ, Epner PL, Ebert C et al. . IFCC Task Force on the Impact of Laboratory Medicine on Clinical Management and Outcomes Current evidence and future perspectives on the effective practice of patient-centered laboratory medicine. Clin Chem. 2015;61:589-599. - PubMed
-
- Plebani M. Towards a new paradigm in laboratory medicine: the five rights. Clin Chem Lab Med. 2016;54:1881-1891. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

