Efficacy of Vedolizumab in Fistulising Crohn's Disease: Exploratory Analyses of Data from GEMINI 2
- PMID: 29471381
- PMCID: PMC6018899
- DOI: 10.1093/ecco-jcc/jjy019
Efficacy of Vedolizumab in Fistulising Crohn's Disease: Exploratory Analyses of Data from GEMINI 2
Abstract
Background and aims: Medical management of fistulising Crohn's disease [CD] is constrained by the limited number of available therapies. We evaluated the efficacy of vedolizumab, a gut-selective α4β7 integrin antagonist approved for treating moderately to severely active CD, in a subpopulation of patients with fistulising CD who participated in the GEMINI 2 trial [NCT00783692].
Methods: Exploratory analyses of data from the GEMINI 2 trial were conducted in 461 responders to 6-week vedolizumab induction therapy who received maintenance placebo [VDZ/PBO, N = 153] or vedolizumab [VDZ/VDZ, N = 308]. Fistula closure rates were assessed at Weeks 14 and 52, and the time to fistula closure was analysed by the Cox proportional hazards model with adjustments for significant covariates.
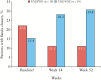
Results: At entry into the maintenance period, 153 [33%] patients had a history of fistulising disease and 57 [12%] patients had ≥1 active draining fistula. By Week 14, 28% of VDZ/VDZ-treated patients compared with 11% of VDZ/PBO-treated patients (95% confidence interval [CI], -11.4 to 43.9) achieved fistula closure. Corresponding rates at Week 52 were 31% and 11% (absolute risk reduction [ARR]: 19.7%; 95% CI, -8.9 to 46.2). Similarly, VDZ/VDZ-treated patients had faster time to fistula closure and were more likely to have fistula closure at Week 52 [33% vs 11%; HR: 2.54; 95% CI, 0.54-11.96]. Prior failure of antibiotic therapy was a negative predictor of fistula closure [HR: 0.217; 95% CI, 0.059-0.795; p = 0.021], whereas trough vedolizumab concentrations did not affect closure rates.
Conclusions: Our findings are consistent with the beneficial effect of vedolizumab treatment for fistulising CD.
Figures

References
-
- Vavricka SR, Rogler G. Fistula treatment: the unresolved challenge. Dig Dis 2010;28:556–64. - PubMed
-
- Cosnes J, Cattan S, Blain A et al. . Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis 2002;8:244–50. - PubMed
-
- Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet 2017;389:1741–55. - PubMed
-
- Gecse KB, Bemelman W, Kamm MA et al. ; World Gastroenterology Organization, International Organisation for Inflammatory Bowel Diseases IOIBD, European Society of Coloproctology and Robarts Clinical Trials A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut 2014;63:1381–92. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

