Mechanisms Underpinning the Polypharmacy Effects of Medications in Psychiatry
- PMID: 29471411
- PMCID: PMC6007392
- DOI: 10.1093/ijnp/pyy014
Mechanisms Underpinning the Polypharmacy Effects of Medications in Psychiatry
Abstract
Background: Bipolar disorder is a mental health condition with progressive social and cognitive function disturbances. Most patients' treatments are based on polypharmacy, but with no biological basis and little is known of the drugs' interactions. The aim of this study was to analyze the effects of lithium, valproate, quetiapine, and lamotrigine, and the interactions between them, on markers of inflammation, bioenergetics, mitochondrial function, and oxidative stress in neuron-like cells and microglial cells.
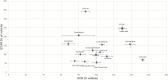
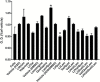
Methods: Neuron-like cells and lipopolysaccharide-stimulated C8-B4 cells were treated with lithium (2.5 mM), valproate (0.5 mM), quetiapine (0.05 mM), and lamotrigine (0.05 mM) individually and in all possible combinations for 24 h. Twenty cytokines were measured in the media from lipopolysaccharide-stimulated C8-B4 cells. Metabolic flux analysis was used to measure bioenergetics, and real-time PCR was used to measure the expression of mitochondrial function genes in neuron-like cells. The production of superoxide in treated cells was also assessed.
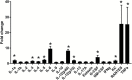
Results: The results suggest major inhibitory effects on proinflammatory cytokine release as a therapeutic mechanism of these medications when used in combination. The various combinations of medications also caused overexpression of PGC1α and ATP5A1 in neuron-like cells. Quetiapine appears to have a proinflammatory effect in microglial cells, but this was reversed by the addition of lamotrigine independent of the drug combination.
Conclusion: Polypharmacy in bipolar disorder may have antiinflammatory effects on microglial cells as well as effects on mitochondrial biogenesis in neuronal cells.
Figures

References
-
- Adli M, Whybrow PC, Grof P, Rasgon N, Gyulai L, Baethge C, Glenn T, Bauer M(2005)Use of polypharmacy and self-reported mood in outpatients with bipolar disorder. Int J Psychiatry Clin Pract 9:251–256. - PubMed
-
- Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Yücel M, Gama CS, Dodd S, Dean B, Magalhães PV, Amminger P, McGorry P, Malhi GS(2011)Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev 35:804–817. - PubMed
-
- Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, Ganie SA(2015)Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother 74:101–110. - PubMed
-
- Block ML, Zecca L, Hong JS(2007)Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69. - PubMed
-
- Caliyurt O, Altiay G(2009)Resting energy expenditure in manic episode. Bipolar Disord 11:102–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

