rA1M-035, a Physicochemically Improved Human Recombinant α1-Microglobulin, Has Therapeutic Effects in Rhabdomyolysis-Induced Acute Kidney Injury
- PMID: 29471681
- PMCID: PMC6338582
- DOI: 10.1089/ars.2017.7181
rA1M-035, a Physicochemically Improved Human Recombinant α1-Microglobulin, Has Therapeutic Effects in Rhabdomyolysis-Induced Acute Kidney Injury
Erratum in
-
Correction to: rA1M-035, a Physicochemically Improved Human Recombinant α1-Microglobulin, Has Therapeutic Effects in Rhabdomyolysis-Induced Acute Kidney Injury by Åkerström B, Rosenlöf L, Hägerwall A, Rutardottir S, Ahlstedt J, Johansson ME, Erlandsson L, Allhorn M, and Gram M. Antioxidants & Redox Signaling 30: 489-504, 2019. DOI: 10.1089/ars.2017.7181.Antioxid Redox Signal. 2020 Mar 20;32(9):656. doi: 10.1089/ars.2017.7181.correx. Antioxid Redox Signal. 2020. PMID: 32069103 Free PMC article. No abstract available.
Abstract
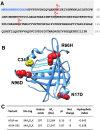
Aims: Human α1-microglobulin (A1M) is an endogenous reductase and radical- and heme-binding protein with physiological antioxidant protective functions. Recombinant human A1M (rA1M) has been shown to have therapeutic properties in animal models of preeclampsia, a pregnancy disease associated with oxidative stress. Recombinant A1M, however, lacks glycosylation, and shows lower solubility and stability than A1M purified from human plasma. The aims of this work were to (i) use site-directed mutagenesis to improve the physicochemical properties of rA1M, (ii) demonstrate that the physicochemically improved rA1M displays full in vitro cell protective effects as recombinant wild-type A1M (rA1M-wt), and (iii) show its therapeutic potential in vivo against acute kidney injury (AKI), another disease associated with oxidative stress.
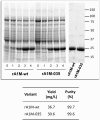
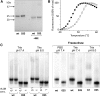
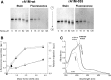
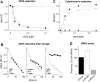
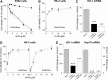
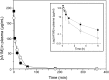
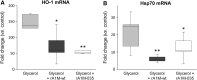
Results: A novel recombinant A1M-variant (rA1M-035) with three amino acid substitutions was constructed, successfully expressed, and purified. rA1M-035 had improved solubility and stability compared with rA1M-wt, and showed intact in vitro heme-binding, reductase, antioxidation, and cell protective activities. Both rA1M-035 and rA1M-wt showed, for the first time, potential in vivo protective effects on kidneys using a mouse rhabdomyolysis glycerol injection model of AKI.
Innovation: A novel recombinant A1M-variant, rA1M-035, was engineered. This protein showed improved solubility and stability compared with rA1M-wt, full in vitro functional activity, and potential protection against AKI in an in vivo rhabdomyolysis mouse model.
Conclusion: The new rA1M-035 is a better drug candidate than rA1M-wt for treatment of AKI and preeclampsia in human patients.
Keywords: acute kidney injury; alpha-1-microglobulin; antioxidant; heme-binding; radical scavenger; reductase.
Conflict of interest statement
The authors B.Å., L.R., A.H., J.A., and M.G. are shareholders of the company A1M Pharma, which holds patents related to A1M usage and production. This does not present any conflict of interest.
Figures

References
-
- Åkerström B, Borregaard N, Flower D, and Salier J-P, eds. Lipocalins. Georgetown, TX: Landes Bioscience, 2006
-
- Åkerström B. and Gram M. A1M, an extravascular tissue cleaning and housekeeping protein. Free Radic Biol Med 74: 274–282, 2014 - PubMed
-
- Åkerström B. and Lögdberg L. An intriguing member of the lipocalin protein family: α1-microglobulin. Trends Biochem Sci 15: 240–243, 1990 - PubMed
-
- Åkerström B, Maghzal G, Winterbourn CC, and Kettle AJ. The lipocalin α1-microglobulin has radical-scavenger activity. J Biol Chem 282: 31493–31503, 2007 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
