Large Animal Models of an In Vivo Bioreactor for Engineering Vascularized Bone
- PMID: 29471732
- PMCID: PMC6080121
- DOI: 10.1089/ten.TEB.2018.0005
Large Animal Models of an In Vivo Bioreactor for Engineering Vascularized Bone
Abstract
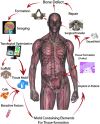
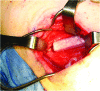
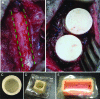
Reconstruction of large skeletal defects is challenging due to the requirement for large volumes of donor tissue and the often complex surgical procedures. Tissue engineering has the potential to serve as a new source of tissue for bone reconstruction, but current techniques are often limited in regards to the size and complexity of tissue that can be formed. Building tissue using an in vivo bioreactor approach may enable the production of appropriate amounts of specialized tissue, while reducing issues of donor site morbidity and infection. Large animals are required to screen and optimize new strategies for growing clinically appropriate volumes of tissues in vivo. In this article, we review both ovine and porcine models that serve as models of the technique proposed for clinical engineering of bone tissue in vivo. Recent findings are discussed with these systems, as well as description of next steps required for using these models, to develop clinically applicable tissue engineering applications.
Keywords: bone regeneration; large animal models; ovine model; periosteum; porcine model.
Conflict of interest statement
No competing financial interests exist.
Figures





References
-
- Foster R.D., Anthony J.P., Sharma A., and Pogrel M.A. Vascularized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: an outcome analysis of primary bony union and endosseous implant success. Head Neck 21, 66, 1999 - PubMed
-
- McCullen S.D., Chow A.G., and Stevens M.M. In vivo tissue engineering of musculoskeletal tissues. Curr Opin Biotechnol 22, 715, 2011 - PubMed
-
- Yang Y., Hallgrimsson B., and Putnins E.E. Craniofacial defect regeneration using engineered bone marrow mesenchymal stromal cells. J Biomed Mater Res Part A 99A, 74, 2011 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

