Prediction of suboptimal cytoreductive surgery in patients with advanced ovarian cancer based on preoperative and intraoperative determination of the peritoneal carcinomatosis index
- PMID: 29471831
- PMCID: PMC5824576
- DOI: 10.1186/s12957-018-1339-0
Prediction of suboptimal cytoreductive surgery in patients with advanced ovarian cancer based on preoperative and intraoperative determination of the peritoneal carcinomatosis index
Abstract
Background: The peritoneal carcinomatosis index (PCI) can be used to quantify the tumor burden in patients with advanced ovarian cancer. The aim of the present study was to establish a predictive model for suboptimal cytoreductive surgery (SCS) (residual tumor of > 1 cm) using preoperative and intraoperative determination of the PCI.
Methods: In total, 110 consecutive patients treated for advanced ovarian cancer during a 4-year period in our institution were assessed. Eighty of these patients were eligible for primary debulking surgery and thus included in the present study. All data were prospectively collected and retrospectively evaluated. We determined the PCI both preoperatively and intraoperatively and assessed postoperative complications.
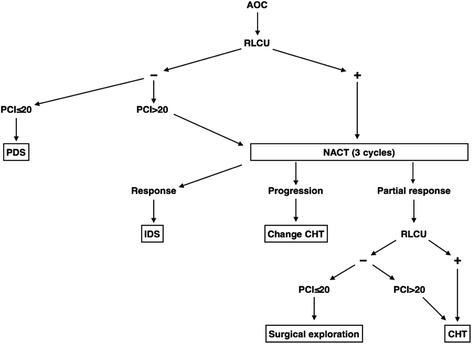
Results: A PCI of > 20 was the best cut-off with which to predict a risk of SCS among all three diagnostic techniques assessed in this study (computed tomography, laparoscopy, and laparotomy). Intraoperative PCI determination was associated with the lowest risk of false negatives for SCS when detecting a PCI of < 20. The combination of preoperative computed tomography and laparoscopy, when both techniques predicted SCS, was associated with the lowest risk of false positives for SCS when detecting a PCI of > 20.
Conclusion: The combination of computed tomography and laparoscopy to obtain the PCI can help to determine which patients with advanced ovarian cancer are suitable for primary debulking surgery and which should undergo neoadjuvant chemotherapy.
Keywords: Advanced ovarian cancer; Cytoreductive surgery; Peritoneal carcinomatosis index.
Conflict of interest statement
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the local ethics and research committee and followed the Declaration of Helsinki guidelines. Written informed consent was required for collecting data.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Llueca JA, Martinez-Ramos D, Escrig-Sos J, Torrella-Ramos A, Herraiz JL, et al. Current status of ovarian cancer in the Spanish Province of Castellon. Prognostic factors in observed and relative survival. A population cancer registry-based study between 2004 and 2008. Prog Obstet Ginecol. 2014;57:405–412. doi: 10.1016/j.pog.2014.05.007. - DOI
-
- Ledermann FA JA, Raja C, Fotopoulou A, Gonzalez-Martin N, Colombo C, Sessa C, ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013; 10.1093/annonc/mdt333. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

