Controlled Education of patients after Stroke (CEOPS)- nurse-led multimodal and long-term interventional program involving a patient's caregiver to optimize secondary prevention of stroke: study protocol for a randomized controlled trial
- PMID: 29471839
- PMCID: PMC5824577
- DOI: 10.1186/s13063-018-2483-0
Controlled Education of patients after Stroke (CEOPS)- nurse-led multimodal and long-term interventional program involving a patient's caregiver to optimize secondary prevention of stroke: study protocol for a randomized controlled trial
Abstract
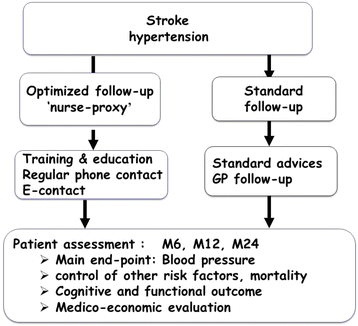
Background: Setting up a follow-up secondary prevention program after stroke is difficult due to motor and cognitive impairment, but necessary to prevent recurrence and improve patients' quality of life. To involve a referent nurse and a caregiver from the patient's social circle in nurse-led multimodal and long-term management of risk factors after stroke could be an advantage due to their easier access to the patient and family. The aim of this study is to compare the benefit of optimized follow up by nursing personnel from the vascular neurology department including therapeutic follow up, and an interventional program directed to the patient and a caregiving member of their social circle, as compared with typical follow up in order to develop a specific follow-up program of secondary prevention of stroke.
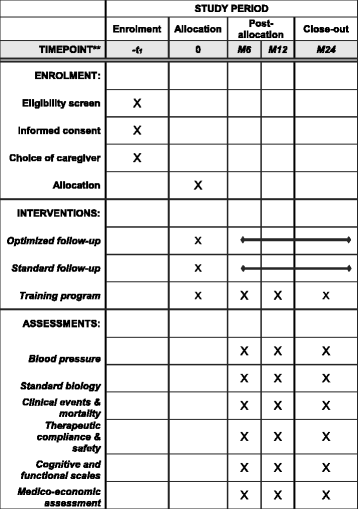
Methods/design: The design is a randomized, controlled, clinical trial conducted in the French Stroke Unit of the Strokavenir network. In total, 410 patients will be recruited and randomized in optimized follow up or usual follow up for 2 years. In both group, patients will be seen by a neurologist at 6, 12 and 24 months. The optimized follow up will include follow up by a nurse from the vascular neurology department, including therapeutic follow up, and a training program on secondary prevention directed to the patient and a caregiving member of their social circle. After discharge, a monthly telephone interview, in the first year and every 3 months in the second year, will be performed by the nurse. At 6, 12 and 24 month, the nurse will give the patient and caregiver another training session. Usual follow up is only done by the patient's general practitioner, after classical information on secondary prevention of risk factors during hospitalization. The primary outcome measure is blood pressure measured after the first year of follow up. Blood pressure will be measured by nursing personnel who do not know the group into which the patient has been randomized. Secondary endpoints are associated mortality, morbidity, recurrence, drug side-effects and medico-economic analysis.
Discussion: The result of this trial is expected to provide the benefit of a nurse-led optimized multimodal and long-term interventional program for management of risk factors after stroke, personalizing the role of the nurse and including the patient's caregiver.
Trial registration: ClinicalTrials.gov, NCT 02132364. Registered on 7 May 2014. EUDRACT, A 00473-40.
Trial registration: ClinicalTrials.gov NCT02132364.
Keywords: Family caregiver; Multimodal intervention; Nurse; Secondary prevention; Stroke.
Conflict of interest statement
Ethics approval and consent to participate
The protocol has been approved by CPP Nord-Ouest IV on 2012 (A00473 40) with 11 approved modifications (in particular for recruitment of new centers). The last version of the protocol was approved on 23 may 2017. All patients and caregivers have to give their consent before inclusion.
Consent for publication
The authors have been informed of the publication and gave their consent.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil-Smoller S, Turan TN, Wentworth D, on behalf of the American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. - DOI - PubMed
-
- Brainin M, Matz K, Nemec M, Teuschl Y, Dachenhausen A, Asenbaum-Nan S, Bancher C, Kepplinger B, Oberndorfer S, Pinter M, Schnider P, Tuomilehto J, ASPIS Study Group Prevention of poststroke cognitive decline: ASPIS–a multicenter, randomized, observer-blind, parallel group clinical trial to evaluate multiple lifestyle interventions–study design and baseline characteristics. Int J Stroke. 2015;10(4):627–635. doi: 10.1111/ijs.12188. - DOI - PubMed
-
- Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC, Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S76–S99. doi: 10.1161/01.cir.0000437740.48606.d1. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical