TNFα and Radioresistant Stromal Cells Are Essential for Therapeutic Efficacy of Cyclic Dinucleotide STING Agonists in Nonimmunogenic Tumors
- PMID: 29472271
- PMCID: PMC6215542
- DOI: 10.1158/2326-6066.CIR-17-0263
TNFα and Radioresistant Stromal Cells Are Essential for Therapeutic Efficacy of Cyclic Dinucleotide STING Agonists in Nonimmunogenic Tumors
Abstract
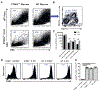
The cGAS-STING cytosolic DNA sensing pathway may play an integral role in the initiation of antitumor immune responses. Studies evaluating the immunogenicity of various cyclic dinucleotide (CDN) STING agonists administered by intratumoral (i.t.) injection showed potent induction of inflammation, tumor necrosis, and, in some cases, durable tumor-specific adaptive immunity. However, the specific immune mechanisms underlying these responses remain incompletely defined. The majority of these studies have focused on the effect of CDNs on immune cells but have not conclusively interrogated the role of stromal cells in the acute rejection of the CDN-injected tumor. Here, we revealed a mechanism of STING agonist-mediated tumor response that relied on both stromal and immune cells to achieve tumor regression and clearance. Using knockout and bone marrow chimeric mice, we showed that although bone marrow-derived TNFα was necessary for CDN-induced necrosis, STING signaling in radioresistant stromal cells was also essential for CDN-mediated tumor rejection. These results provide evidence for crosstalk between stromal and hematopoietic cells during CDN-mediated tumor collapse after i.t. administration. These mechanistic insights may prove critical in the clinical development of STING agonists. Cancer Immunol Res; 6(4); 422-33. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
A.L. Desbien is a scientist at Aduro Biotech and reports receiving a commercial research grant from the same. K.E. Sivick has ownership interest in a patent with Aduro Biotech. C.G. Drake reports receiving a commercial research grant from Aduro Biotech. No potential conflicts of interest were disclosed by the other authors.
Figures



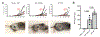

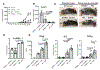
References
-
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001;410:1099–103. - PubMed
-
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J Immunol 1999;162:3749–52. - PubMed
-
- O’Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors redefining innate immunity. Nat Rev Immunol 2013;13:453–60. - PubMed
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 2004;4:499–511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

