Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still's disease
- PMID: 29472362
- PMCID: PMC5965361
- DOI: 10.1136/annrheumdis-2017-212608
Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still's disease
Abstract
Objectives: Adult-onset Still's disease (AOSD) is a rare systemic autoinflammatory disease; its management is largely empirical. This is the first clinical study to determine if interleukin (IL)-18 inhibition, using the recombinant human IL-18 binding protein, tadekinig alfa, is a therapeutic option in AOSD.
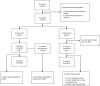
Methods: In this phase II, open-label study, patients were ≥18 years with active AOSD plus fever or C reactive protein (CRP) levels ≥10 mg/L despite treatment with prednisone and/or conventional synthetic disease-modifying antirheumatic drugs (DMARDs). Previous biological DMARD treatment was permitted. Patients received tadekinig alfa 80 mg or 160 mg subcutaneously three times per week for 12 weeks; those receiving 80 mg not achieving early predicted response criteria (reduction of ≥50% CRP values from baseline and fever resolution) were up-titrated to 160 mg for a further 12 weeks. The primary endpoint was the occurrence of adverse events (AEs) throughout the study.
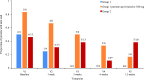
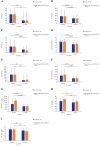
Results: Ten patients were assigned to receive 80 mg tadekinig alfa and 13 patients to the 160 mg dose. One hundred and fifty-five treatment-emerging AEs were recorded, and 47 were considered related to the study drug. Most AEs were mild and resolved after drug discontinuation. Three serious AEs occurred, one possibly related to treatment (toxic optic neuropathy). At week 3, 5 of 10 patients receiving 80 mg and 6 of 12 patients receiving 160 mg achieved the predefined response criteria.
Conclusions: Our results indicate that tadekinig alfa appears to have a favourable safety profile and is associated with early signs of efficacy in patients with AOSD.
Trial registration number: NCT02398435.
Keywords: adult onset still’s disease; inflammation; juvenile idiopathic arthritis.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: CG has received grants and personal fees from AB2 Bio Ltd, grants and personal fees from Roche and Pfizer, and personal fees from Merck Sharp & Dohme (MSD), Novartis, Sanofi and AbbVie; BF has received grants from AbbVie, MSD, Pfizer and Roche, and personal fees from AbbVie, Biogen, Bristol-Myers Squibb (BMS), Celgene, Janssen, Lilly, MSD, MEDAC, Nordic, Pfizer, Roche, Swedish Orphan Biovitrum AB (publ) (Sobi), Novartis and Union Chimique Belge (UCB); FS has received grants from AB2 Bio Ltd; EF has received financial and non-financial support from AB2 Bio Ltd; IK has received personal fees from AbbVie, Actelion, BMS, Celgene, GlaxoSmithKline, Janssen, Novartis, Pfizer, Roche and Sobi; TS has received grants and personal fees from Pfizer and Roche, and personal fees from AbbVie, Biogen, BMS, Lilly, MSD, Novartis, Sanofi and UCB; BH has received personal fees from AbbVie, BMS, Novartis, Roche, Pfizer, Celgene and MSD; PL has received non-financial support from AB2 Bio Ltd; DSC has received grants from AB2 Bio Ltd and personal fees from Pfizer, BMS and Janssen; AS and EJS are employees of AB2 Bio Ltd.
Figures
Comment in
-
Therapeutic innovation in adult-onset Still's disease (and other rare inflammatory disorders): how to secure evidence-based medicine?Ann Rheum Dis. 2018 Dec;77(12):1699-1701. doi: 10.1136/annrheumdis-2018-213106. Epub 2018 Jun 2. Ann Rheum Dis. 2018. PMID: 29860231 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

