Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin
- PMID: 29472541
- PMCID: PMC5823889
- DOI: 10.1038/s41467-018-03124-z
Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin
Abstract
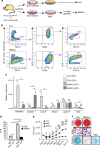
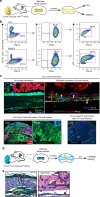
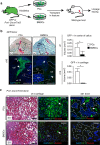
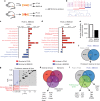
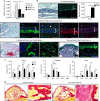
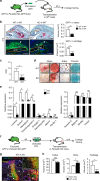
Bone regeneration relies on the activation of skeletal stem cells (SSCs) that still remain poorly characterized. Here, we show that periosteum contains SSCs with high bone regenerative potential compared to bone marrow stromal cells/skeletal stem cells (BMSCs) in mice. Although periosteal cells (PCs) and BMSCs are derived from a common embryonic mesenchymal lineage, postnatally PCs exhibit greater clonogenicity, growth and differentiation capacity than BMSCs. During bone repair, PCs can efficiently contribute to cartilage and bone, and integrate long-term after transplantation. Molecular profiling uncovers genes encoding Periostin and other extracellular matrix molecules associated with the enhanced response to injury of PCs. Periostin gene deletion impairs PC functions and fracture consolidation. Periostin-deficient periosteum cannot reconstitute a pool of PCs after injury demonstrating the presence of SSCs within periosteum and the requirement of Periostin in maintaining this pool. Overall our results highlight the importance of analyzing periosteum and PCs to understand bone phenotypes.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Hall, B. K. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. (Elsevier Academic Press, 2005).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

