Inhibitory modulation of cytochrome c oxidase activity with specific near-infrared light wavelengths attenuates brain ischemia/reperfusion injury
- PMID: 29472564
- PMCID: PMC5823933
- DOI: 10.1038/s41598-018-21869-x
Inhibitory modulation of cytochrome c oxidase activity with specific near-infrared light wavelengths attenuates brain ischemia/reperfusion injury
Erratum in
-
Publisher Correction: Inhibitory modulation of cytochrome c oxidase activity with specific near-infrared light wavelengths attenuates brain ischemia/reperfusion injury.Sci Rep. 2018 Apr 25;8(1):6729. doi: 10.1038/s41598-018-25184-3. Sci Rep. 2018. PMID: 29695825 Free PMC article.
Abstract
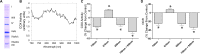
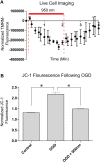
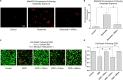
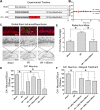
The interaction of light with biological tissue has been successfully utilized for multiple therapeutic purposes. Previous studies have suggested that near infrared light (NIR) enhances the activity of mitochondria by increasing cytochrome c oxidase (COX) activity, which we confirmed for 810 nm NIR. In contrast, scanning the NIR spectrum between 700 nm and 1000 nm revealed two NIR wavelengths (750 nm and 950 nm) that reduced the activity of isolated COX. COX-inhibitory wavelengths reduced mitochondrial respiration, reduced the mitochondrial membrane potential (ΔΨm), attenuated mitochondrial superoxide production, and attenuated neuronal death following oxygen glucose deprivation, whereas NIR that activates COX provided no benefit. We evaluated COX-inhibitory NIR as a potential therapy for cerebral reperfusion injury using a rat model of global brain ischemia. Untreated animals demonstrated an 86% loss of neurons in the CA1 hippocampus post-reperfusion whereas inhibitory NIR groups were robustly protected, with neuronal loss ranging from 11% to 35%. Moreover, neurologic function, assessed by radial arm maze performance, was preserved at control levels in rats treated with a combination of both COX-inhibitory NIR wavelengths. Taken together, our data suggest that COX-inhibitory NIR may be a viable non-pharmacologic and noninvasive therapy for the treatment of cerebral reperfusion injury.
Conflict of interest statement
T.H.S. and M.H. are co-founders of Mitovation Inc. that develops infrared light therapy for ischemia/reperfusion injury applications. All other authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Toledo-Pereyra LH, Lopez-Neblina F, Toledo AH. Reactive oxygen species and molecular biology of ischemia/reperfusion. Ann Transplant. 2004;9:81–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

