Polyrotaxane-based supramolecular theranostics
- PMID: 29472567
- PMCID: PMC5823937
- DOI: 10.1038/s41467-018-03119-w
Polyrotaxane-based supramolecular theranostics
Abstract
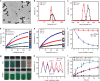
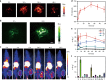
The development of smart theranostic systems with favourable biocompatibility, high loading efficiency, excellent circulation stability, potent anti-tumour activity, and multimodal diagnostic functionalities is of importance for future clinical application. The premature burst release and poor degradation kinetics indicative of polymer-based nanomedicines remain the major obstacles for clinical translation. Herein we prepare theranostic shell-crosslinked nanoparticles (SCNPs) using a β-cyclodextrin-based polyrotaxane (PDI-PCL-b-PEG-RGD⊃β-CD-NH2) to avoid premature drug leakage and achieve precisely controllable release, enhancing the maximum tolerated dose of the supramolecular nanomedicines. cRGDfK and perylene diimide are chosen as the stoppers of PDI-PCL-b-PEG-RGD⊃β-CD-NH2, endowing the resultant SCNPs with excellent integrin targeting ability, photothermal effect, and photoacoustic capability. In vivo anti-tumour studies demonstrate that drug-loaded SCNPs completely eliminate the subcutaneous tumours without recurrence after a single-dose injection combining chemotherapy and photothermal therapy. These supramolecular nanomedicines also exhibit excellent anti-tumour performance against orthotopic breast cancer and prevent lung metastasis with negligible systemic toxicity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

