Role of trace amine-associated receptor 1 in nicotine's behavioral and neurochemical effects
- PMID: 29472642
- PMCID: PMC6180004
- DOI: 10.1038/s41386-018-0017-9
Role of trace amine-associated receptor 1 in nicotine's behavioral and neurochemical effects
Abstract
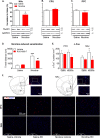
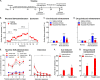
Nicotine addiction and abuse remains a global health issue. To date, the fundamental neurobiological mechanism of nicotine addiction remains incompletely understood. Trace amine-associated receptor 1 (TAAR1) is thought to directly modulate dopaminergic system and are thought to be a neural substrate underlying addictive-like behaviors. We aimed to investigate the role of TAAR1 in nicotine addictive-like behaviors. TAAR1 expression after nicotine treatment was evaluated by western blotting. c-Fos immunofluorescence and in vivo fast-scan cyclic voltammetry were used to examine the activation of brain regions and dopamine release, respectively. We then thoroughly and systematically examined the role of TAAR1 in mediating nicotine-induced sensitization, nicotine discrimination, nicotine self-administration, nicotine demand curve, and the reinstatement of nicotine-seeking. Local pharmacological manipulation was conducted to determine the role of TAAR1 in the nucleus accumbens (NAcs) in the reinstatement of nicotine-seeking. We found that the expression of TAAR1 protein was selectively downregulated in the NAc, with no change in either dorsal striatum or prefrontal cortex. TAAR1 activation was sufficient to block nicotine-induced c-Fos expression in the NAc, while also reducing nicotine-induced dopamine release in the NAc. Systemic administration of TAAR1 agonists attenuated the expression and development of nicotine-induced sensitization, nicotine self-administration, the reinstatement of nicotine-seeking, and increased the elasticity of nicotine demand curve, while intra-NAc infusions of a TAAR1 agonist was sufficient to attenuate nicotine reinstatement. Moreover, TAAR1-knockout rats showed augmented cue-induced and drug-induced reinstatement of nicotine-seeking. These results indicated that modulation of TAAR1 activity regulates nicotine addictive-like behaviors and TAAR1 represents a novel target towards the treatment of nicotine addiction.
Conflict of interest statement
MCH is a current employee of F Hoffmann-La Roche Ltd. F Hoffmann-La Roche Ltd plays no role in the experiments and interpretation of the data. The authors declare that they have no conflict of interest.
Figures



Similar articles
-
TAAR1 and Psychostimulant Addiction.Cell Mol Neurobiol. 2020 Mar;40(2):229-238. doi: 10.1007/s10571-020-00792-8. Epub 2020 Jan 23. Cell Mol Neurobiol. 2020. PMID: 31974906 Free PMC article. Review.
-
Role of TAAR1 within the Subregions of the Mesocorticolimbic Dopaminergic System in Cocaine-Seeking Behavior.J Neurosci. 2017 Jan 25;37(4):882-892. doi: 10.1523/JNEUROSCI.2006-16.2016. J Neurosci. 2017. PMID: 28123023 Free PMC article.
-
A partial trace amine-associated receptor 1 agonist exhibits properties consistent with a methamphetamine substitution treatment.Addict Biol. 2017 Sep;22(5):1246-1256. doi: 10.1111/adb.12410. Epub 2016 May 19. Addict Biol. 2017. PMID: 27193165
-
TAAR1 regulates drug-induced reinstatement of cocaine-seeking via negatively modulating CaMKIIα activity in the NAc.Mol Psychiatry. 2022 Apr;27(4):2136-2145. doi: 10.1038/s41380-022-01448-3. Epub 2022 Jan 25. Mol Psychiatry. 2022. PMID: 35079125 Free PMC article.
-
TAAR1 in Addiction: Looking Beyond the Tip of the Iceberg.Front Pharmacol. 2018 Mar 27;9:279. doi: 10.3389/fphar.2018.00279. eCollection 2018. Front Pharmacol. 2018. PMID: 29636691 Free PMC article. Review.
Cited by
-
Interleukin-1 receptor-associated kinase 4 (IRAK4) in the nucleus accumbens regulates opioid-seeking behavior in male rats.Brain Behav Immun. 2022 Mar;101:37-48. doi: 10.1016/j.bbi.2021.12.014. Epub 2021 Dec 24. Brain Behav Immun. 2022. PMID: 34958862 Free PMC article.
-
Trace Amine-Associated Receptors' Role in Immune System Functions.Biomedicines. 2024 Apr 18;12(4):893. doi: 10.3390/biomedicines12040893. Biomedicines. 2024. PMID: 38672247 Free PMC article. Review.
-
TAAR1 and Psychostimulant Addiction.Cell Mol Neurobiol. 2020 Mar;40(2):229-238. doi: 10.1007/s10571-020-00792-8. Epub 2020 Jan 23. Cell Mol Neurobiol. 2020. PMID: 31974906 Free PMC article. Review.
-
The Action of TAAR1 Agonist RO5263397 on Executive Functions in Rats.Cell Mol Neurobiol. 2020 Mar;40(2):215-228. doi: 10.1007/s10571-019-00757-6. Epub 2019 Nov 16. Cell Mol Neurobiol. 2020. PMID: 31734895 Free PMC article.
-
TAAR Agonists.Cell Mol Neurobiol. 2020 Mar;40(2):257-272. doi: 10.1007/s10571-019-00774-5. Epub 2019 Dec 17. Cell Mol Neurobiol. 2020. PMID: 31848873 Free PMC article. Review.
References
-
- World Health Organization. WHO report on the global tobacco epidemic. Geneva, Switzerland: World Health Organization; 2011.
-
- Leri F, Vaccarino FJ. Tribute to: self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area [William Corrigall, Kathleen Coen and Laurel Adamson. Brain Res. 653 (1994) 278–284] Brain Res. 2016;1645:61–64. doi: 10.1016/j.brainres.2015.12.064. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

