Interaction between noradrenergic and cholinergic signaling in amygdala regulates anxiety- and depression-related behaviors in mice
- PMID: 29472646
- PMCID: PMC6098039
- DOI: 10.1038/s41386-018-0024-x
Interaction between noradrenergic and cholinergic signaling in amygdala regulates anxiety- and depression-related behaviors in mice
Abstract
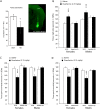
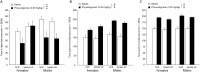
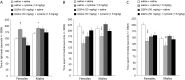
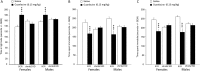
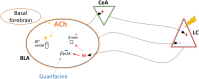
Medications that target the noradrenergic system are important therapeutics for depression and anxiety disorders. More recently, clinical studies have shown that the α2-noradrenergic receptor (α2AR) agonist guanfacine can decrease stress-induced smoking relapse during acute abstinence, suggesting that targeting the noradrenergic system may aid in smoking cessation through effects on stress pathways in the brain. Acetylcholine (ACh), like the nicotine in tobacco, acts at nicotinic acetylcholine receptors (nAChRs) to regulate behaviors related to anxiety and depression. We therefore investigated interactions between guanfacine and ACh signaling in tests of anxiolytic and antidepressant efficacy in female and male C57BL/6J mice, focusing on the amygdala as a potential site of noradrenergic/cholinergic interaction. The antidepressant-like effects of guanfacine were blocked by shRNA-mediated knockdown of α2AR in amygdala. Knockdown of the high-affinity β2 nAChR subunit in amygdala also prevented antidepressant-like effects of guanfacine, suggesting that these behavioral effects require ACh signaling through β2-containing nAChRs in this brain area. Ablation of NE terminals prevented the anxiolytic- and antidepressant-like effects of the nicotinic partial agonist cytisine, whereas administration of the cholinesterase antagonist physostigmine induced a depression-like phenotype that was not altered by knocking down α2AR in the amygdala. These studies suggest that ACh and NE have opposing actions on behaviors related to anxiety and depression and that cholinergic signaling through β2-containing nAChRs and noradrenergic signaling through α2a receptors in neurons of the amygdala are critical for regulation of these behaviors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

