Inhibition of TNFα-interacting protein α (Tipα)-associated gastric carcinogenesis by BTG2/TIS21 via downregulating cytoplasmic nucleolin expression
- PMID: 29472702
- PMCID: PMC5903828
- DOI: 10.1038/emm.2017.281
Inhibition of TNFα-interacting protein α (Tipα)-associated gastric carcinogenesis by BTG2/TIS21 via downregulating cytoplasmic nucleolin expression
Abstract
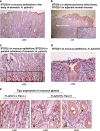
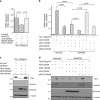
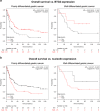
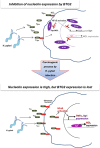
To understand the regulation of Helicobacter pylori (H. pylori)-associated gastric carcinogenesis, we examined the effect of B-cell translocation gene 2 (BTG2) expression on the biological activity of Tipα, an oncoprotein secreted from H. pylori. BTG2, the human ortholog of mouse TIS21 (BTG2/TIS21), has been reported to be a primary response gene that is transiently expressed in response to various stimulations. Here, we report that BTG2 is constitutively expressed in the mucous epithelium and parietal cells of the gastric gland in the stomach. Expression was increased in the mucous epithelium following H. pylori infection in contrast to its loss in human gastric adenocarcinoma. Indeed, adenoviral transduction of BTG2/TIS21 significantly inhibited Tipα activity in MKN-1 and MGT-40, human and mouse gastric cancer cells, respectively, thereby downregulating tumor necrosis factor-α (TNFα) expression and Erk1/2 phosphorylation by reducing expression of nucleolin, a Tipα receptor. Chromatin immunoprecipitation proved that BTG2/TIS21 inhibited Sp1 expression and its binding to the promoter of the nucleolin gene. In addition, BTG2/TIS21 expression significantly reduced membrane-localized nucleolin expression in cancer cells, and the loss of BTG2/TIS21 expression induced cytoplasmic nucleolin availability in gastric cancer tissues, as evidenced by immunoblotting and immunohistochemistry. Higher expression of BTG2 and lower expression of nucleolin were accompanied with better overall survival of poorly differentiated gastric cancer patients. This is the first report showing that BTG2/TIS21 inhibits nucleolin expression via Sp1 binding, which might be associated with the inhibition of H. pylori-induced carcinogenesis. We suggest that BTG2/TIS21 is a potential inhibitor of nucleolin in the cytoplasm, leading to inhibition of carcinogenesis after H. pylori infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

