Bile Salt Hydrolase Activities: A Novel Target to Screen Anti- Giardia Lactobacilli?
- PMID: 29472903
- PMCID: PMC5809405
- DOI: 10.3389/fmicb.2018.00089
Bile Salt Hydrolase Activities: A Novel Target to Screen Anti- Giardia Lactobacilli?
Abstract
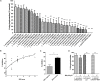
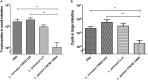
Giardia duodenalis is a protozoan parasite responsible for giardiasis, a disease characterized by intestinal malabsorption, diarrhea and abdominal pain in a large number of mammal species. Giardiasis is one of the most common intestinal parasitic diseases in the world and thus a high veterinary, and public health concern. It is well-established that some probiotic bacteria may confer protection against this parasite in vitro and in vivo and we recently documented the implication of bile-salt hydrolase (BSH)-like activities from strain La1 of Lactobacillus johnsonii as mediators of these effects in vitro. We showed that these activities were able to generate deconjugated bile salts that were toxic to the parasite. In the present study, a wide collection of lactobacilli strains from different ecological origins was screened to assay their anti-giardial effects. Our results revealed that the anti-parasitic effects of some of the strains tested were well-correlated with the expression of BSH-like activities. The two most active strains in vitro, La1 and Lactobacillus gasseri CNCM I-4884, were then tested for their capacity to influence G. duodenalis infection in a suckling mice model. Strikingly, only L. gasseri CNCM I-4884 strain was able to significantly antagonize parasite growth with a dramatic reduction of the trophozoites load in the small intestine. Moreover, this strain also significantly reduced the fecal excretion of Giardia cysts after 5 days of treatment, which could contribute to blocking the transmission of the parasite, in contrast of La1 where no effect was observed. This study represents a step toward the development of new prophylactic strategies to combat G. duodenalis infection in both humans and animals.
Keywords: Giardia duodenalis; Lactobacillus gasseri; Lactobacillus johnsonii; bile salt hydrolases; lactobacilli; probiotics.
Figures

References
-
- Bartelt L. A., Bolick D. T., Mayneris-Perxachs J., Kolling G. L., Medlock G. L., Zaenker E. I., et al. (2017). Cross-modulation of pathogen-specific pathways enhances malnutrition during enteric co-infection with Giardia lamblia and enteroaggregative Escherichia coli. PLOS Pathog. 13:e1006471. 10.1371/journal.ppat.1006471 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

