Novel α-Tubulin Mutations Conferring Resistance to Dinitroaniline Herbicides in Lolium rigidum
- PMID: 29472938
- PMCID: PMC5810296
- DOI: 10.3389/fpls.2018.00097
Novel α-Tubulin Mutations Conferring Resistance to Dinitroaniline Herbicides in Lolium rigidum
Abstract
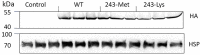
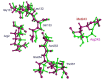
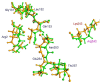
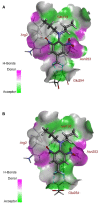
The dinitroaniline herbicides (particularly trifluralin) have been globally used in many crops for selective grass weed control. Consequently, trifluralin resistance has been documented in several important crop weed species and has recently reached a level of concern in Australian Lolium rigidum populations. Here, we report novel mutations in the L. rigidum α-tubulin gene which confer resistance to trifluralin and other dinitroaniline herbicides. Nucleotide mutations at the highly conserved codon Arg-243 resulted in amino acid substitutions of Met or Lys. Rice calli transformed with the mutant 243-Met or 243-Lys α-tubulin genes were 4- to 8-fold more resistant to trifluralin and other dinitroaniline herbicides (e.g., ethalfluralin and pendimethalin) compared to calli transformed with the wild type α-tubulin gene from L. rigidum. Comprehensive modeling of molecular docking predicts that Arg-243 is close to the trifluralin binding site on the α-tubulin surface and that replacement of Arg-243 by Met/Lys-243 results in a spatial shift of the trifluralin binding domain, reduction of trifluralin-tubulin contacts, and unfavorable interactions. The major effect of these substitutions is a significant rise of free interaction energy between α-tubulin and trifluralin, as well as between trifluralin and its whole molecular environment. These results demonstrate that the Arg-243 residue in α-tubulin is a determinant for trifluralin sensitivity, and the novel Arg-243-Met/Lys mutations may confer trifluralin resistance in L. rigidum.
Keywords: Lolium rigidum; dinitroanilines; mutation; trifluralin resistance; α-tubulin.
Figures







Similar articles
-
Dinitroaniline herbicide resistance in a multiple-resistant Lolium rigidum population.Pest Manag Sci. 2018 Apr;74(4):925-932. doi: 10.1002/ps.4790. Epub 2018 Feb 1. Pest Manag Sci. 2018. PMID: 29148165
-
A Val-202-Phe α-tubulin mutation and enhanced metabolism confer dinitroaniline resistance in a single Lolium rigidum population.Pest Manag Sci. 2020 Feb;76(2):645-652. doi: 10.1002/ps.5561. Epub 2019 Aug 15. Pest Manag Sci. 2020. PMID: 31329340
-
Dinitroaniline Herbicide Resistance and Mechanisms in Weeds.Front Plant Sci. 2021 Mar 25;12:634018. doi: 10.3389/fpls.2021.634018. eCollection 2021. Front Plant Sci. 2021. PMID: 33841462 Free PMC article. Review.
-
A dinitroaniline herbicide resistance mutation can be nearly lethal to plants.Pest Manag Sci. 2022 Apr;78(4):1547-1554. doi: 10.1002/ps.6773. Epub 2022 Jan 21. Pest Manag Sci. 2022. PMID: 34981627
-
[Plant mutant tubulin genes as marker selective genes for genetic engineering].Tsitol Genet. 2007 May-Jun;41(3):29-43. Tsitol Genet. 2007. PMID: 17649622 Review. Russian.
Cited by
-
Understanding Resistance Mechanisms to Trifluralin in an Arkansas Palmer Amaranth Population.Genes (Basel). 2021 Aug 10;12(8):1225. doi: 10.3390/genes12081225. Genes (Basel). 2021. PMID: 34440399 Free PMC article.
-
Omics Potential in Herbicide-Resistant Weed Management.Plants (Basel). 2019 Dec 14;8(12):607. doi: 10.3390/plants8120607. Plants (Basel). 2019. PMID: 31847327 Free PMC article. Review.
-
In vitro Ploidy Manipulation for Crop Improvement.Front Plant Sci. 2020 Jun 3;11:722. doi: 10.3389/fpls.2020.00722. eCollection 2020. Front Plant Sci. 2020. PMID: 32582252 Free PMC article. Review.
-
Target-Site Mutations Conferring Herbicide Resistance.Plants (Basel). 2019 Sep 28;8(10):382. doi: 10.3390/plants8100382. Plants (Basel). 2019. PMID: 31569336 Free PMC article. Review.
-
Unveiling the Possible Oryzalin-Binding Site in the α-Tubulin of Toxoplasma gondii.ACS Omega. 2022 May 24;7(22):18434-18442. doi: 10.1021/acsomega.2c00729. eCollection 2022 Jun 7. ACS Omega. 2022. PMID: 35694483 Free PMC article.
References
-
- Abraham M. J., Murtola T., Schulz R., Páll S., Smith J. C., Hess B., et al. (2015). GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1 19–25. 10.1016/j.softx.2015.06.001 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

