Surfactant Protein D in Respiratory and Non-Respiratory Diseases
- PMID: 29473039
- PMCID: PMC5809447
- DOI: 10.3389/fmed.2018.00018
Surfactant Protein D in Respiratory and Non-Respiratory Diseases
Abstract
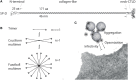
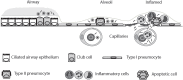
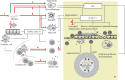
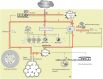
Surfactant protein D (SP-D) is a multimeric collectin that is involved in innate immune defense and expressed in pulmonary, as well as non-pulmonary, epithelia. SP-D exerts antimicrobial effects and dampens inflammation through direct microbial interactions and modulation of host cell responses via a series of cellular receptors. However, low protein concentrations, genetic variation, biochemical modification, and proteolytic breakdown can induce decomposition of multimeric SP-D into low-molecular weight forms, which may induce pro-inflammatory SP-D signaling. Multimeric SP-D can decompose into trimeric SP-D, and this process, and total SP-D levels, are partly determined by variation within the SP-D gene, SFTPD. SP-D has been implicated in the development of respiratory diseases including respiratory distress syndrome, bronchopulmonary dysplasia, allergic asthma, and chronic obstructive pulmonary disease. Disease-induced breakdown or modifications of SP-D facilitate its systemic leakage from the lung, and circulatory SP-D is a promising biomarker for lung injury. Moreover, studies in preclinical animal models have demonstrated that local pulmonary treatment with recombinant SP-D is beneficial in these diseases. In recent years, SP-D has been shown to exert antimicrobial and anti-inflammatory effects in various non-pulmonary organs and to have effects on lipid metabolism and pro-inflammatory effects in vessel walls, which enhance the risk of atherosclerosis. A common SFTPD polymorphism is associated with atherosclerosis and diabetes, and SP-D has been associated with metabolic disorders because of its effects in the endothelium and adipocytes and its obesity-dampening properties. This review summarizes and discusses the reported genetic associations of SP-D with disease and the clinical utility of circulating SP-D for respiratory disease prognosis. Moreover, basic research on the mechanistic links between SP-D and respiratory, cardiovascular, and metabolic diseases is summarized. Perspectives on the development of SP-D therapy are addressed.
Keywords: allergic asthma; atherosclerosis; chronic obstructive lung disease; respiratory distress syndrome; surfactant protein D.
Figures
Similar articles
-
Surfactant protein-D-encoding gene variant polymorphisms are linked to respiratory outcome in premature infants.J Pediatr. 2014 Oct;165(4):683-9. doi: 10.1016/j.jpeds.2014.05.042. Epub 2014 Jul 9. J Pediatr. 2014. PMID: 25015576
-
Surfactant protein A and surfactant protein D variation in pulmonary disease.Immunobiology. 2007;212(4-5):381-416. doi: 10.1016/j.imbio.2007.01.003. Epub 2007 Feb 23. Immunobiology. 2007. PMID: 17544823 Review.
-
Association between the surfactant protein D (SFTPD) gene and subclinical carotid artery atherosclerosis.Atherosclerosis. 2016 Mar;246:7-12. doi: 10.1016/j.atherosclerosis.2015.12.037. Epub 2015 Dec 29. Atherosclerosis. 2016. PMID: 26748346
-
The Dual Role of Surfactant Protein-D in Vascular Inflammation and Development of Cardiovascular Disease.Front Immunol. 2019 Sep 20;10:2264. doi: 10.3389/fimmu.2019.02264. eCollection 2019. Front Immunol. 2019. PMID: 31616435 Free PMC article. Review.
-
Potential of lung surfactant proteins, SP-A and SP-D, and mannan binding lectin for therapy and genetic predisposition to allergic and invasive aspergillosis.Recent Pat Inflamm Allergy Drug Discov. 2007 Nov;1(3):183-7. doi: 10.2174/187221307782418874. Recent Pat Inflamm Allergy Drug Discov. 2007. PMID: 19075981 Review.
Cited by
-
Endothelial Jak3 expression enhances pro-hematopoietic angiocrine function in mice.Commun Biol. 2021 Mar 25;4(1):406. doi: 10.1038/s42003-021-01846-3. Commun Biol. 2021. PMID: 33767339 Free PMC article.
-
Diagnosis of Fibrotic Hypersensitivity Pneumonitis: Is There a Role for Biomarkers?Life (Basel). 2023 Feb 17;13(2):565. doi: 10.3390/life13020565. Life (Basel). 2023. PMID: 36836922 Free PMC article. Review.
-
Association of Surfactant Protein D Single Nucleotide Polymorphisms rs721917, rs2243639, rs3088308 with Recurrent Aphthous Stomatitis in Pakistani Population.Genes (Basel). 2023 May 22;14(5):1119. doi: 10.3390/genes14051119. Genes (Basel). 2023. PMID: 37239479 Free PMC article.
-
Repeated Administration of Clinically Relevant Doses of the Prescription Opioids Tramadol and Tapentadol Causes Lung, Cardiac, and Brain Toxicity in Wistar Rats.Pharmaceuticals (Basel). 2021 Jan 27;14(2):97. doi: 10.3390/ph14020097. Pharmaceuticals (Basel). 2021. PMID: 33513867 Free PMC article.
-
Genetic disorders of the surfactant system: focus on adult disease.Eur Respir Rev. 2021 Feb 16;30(159):200085. doi: 10.1183/16000617.0085-2020. Print 2021 Mar 31. Eur Respir Rev. 2021. PMID: 33597124 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

