Enhancer of zeste homolog 2 (EZH2) inhibitors
- PMID: 29473431
- PMCID: PMC6659997
- DOI: 10.1080/10428194.2018.1430795
Enhancer of zeste homolog 2 (EZH2) inhibitors
Abstract
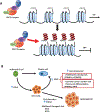
Dysregulation of the histone methyltransferase EZH2 plays a critical role in the development of a variety of malignancies including B-cell lymphomas. As a result, a series of small molecule inhibitors of EZH2 have been developed and studied in the pre-clinical setting. Three EZH2 inhibitors: tazemetostat (EPZ-6438), GSK2816126 and CPI-1205 have moved into phase I/phase II clinical trials in patients with non-Hodgkin lymphoma and genetically defined solid tumors. Early data from the tazemetostat trials indicate an acceptable safety profile and early signs of activity in diffuse large B-cell lymphoma and follicular lymphoma, including patients with EZH2 wild-type and mutant tumors. In this review, we present the rationale, key pre-clinical and early clinical findings of small molecule EZH2 inhibitors for use in lymphoma as well as future challenges and potential opportunities for combination therapies.
Keywords: EZH2 inhibitors; Epigenetic; non-Hodgkin lymphoma.
Figures

References
-
- Strahl BD and Allis CD, The language of covalent histone modifications. Nature, 2000. 403(6765): p. 41–45. - PubMed
-
- Dawson Mark A. and Kouzarides T, Cancer Epigenetics: From Mechanism to Therapy. Cell, 2012. 150(1): p. 12–27. - PubMed
-
- Rodriguez-Paredes M and Esteller M, Cancer epigenetics reaches mainstream oncology. Nat Med, 2011: p. 330–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
