Effect of exenatide QW or placebo, both added to titrated insulin glargine, in uncontrolled type 2 diabetes: The DURATION-7 randomized study
- PMID: 29473704
- PMCID: PMC6032936
- DOI: 10.1111/dom.13266
Effect of exenatide QW or placebo, both added to titrated insulin glargine, in uncontrolled type 2 diabetes: The DURATION-7 randomized study
Abstract
Aims: To compare the efficacy and safety of adding the glucagon-like peptide-1 receptor agonist exenatide once weekly (QW) 2 mg or placebo among patients with type 2 diabetes who were inadequately controlled despite titrated insulin glargine (IG) ± metformin.
Methods: This multicentre, double-blind study (ClinicalTrials.gov identifier: NCT02229383) randomized (1:1) patients with persistent hyperglycaemia after an 8-week titration phase (glycated haemoglobin [HbA1c] 7.0%-10.5% [53-91 mmol/mol]) to exenatide QW or placebo. The primary endpoint was HbA1c change from baseline to week 28. Secondary endpoints included body weight, 2-hour postprandial glucose, and mean daily IG dose.
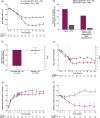
Results: Of 464 randomized patients (mean: age, 58 years; HbA1c, 8.5% [69 mmol/mol]; diabetes duration, 11.3 years), 91% completed 28 weeks. Exenatide QW + IG vs placebo + IG significantly reduced HbA1c (least-squares mean difference, -0.73% [-8.0 mmol/mol]; 95% confidence interval, -0.93%, -0.53% [-10.2, -5.8 mmol/mol]; P < .001; final HbA1c, 7.55% [59 mmol/mol] and 8.24% [67 mmol/mol], respectively); body weight (-1.50 kg; -2.17, -0.84; P < .001); and 2-hour postprandial glucose (-1.52 mmol/L [-27.5 mg/dL]; -2.15, -0.90 [-38.7, -16.2]; P < .001). Significantly more exenatide QW + IG-treated patients vs placebo + IG-treated patients reached HbA1c <7.0% (<53 mmol/mol) (32.5% vs 7.4%; P < .001); daily IG dose increased by 2 and 4 units, respectively. Gastrointestinal and injection-site adverse events were more frequent with exenatide QW + IG (15.1% and 7.8%, respectively) than with placebo + IG (10.8% and 3.0%, respectively); hypoglycaemia incidence was similar between the exenatide QW + IG (29.7%) and placebo + IG (29.0%) groups, with no major hypoglycaemic events.
Conclusions: Among patients with inadequate glycaemic control, exenatide QW significantly improved glucose control and decreased body weight, without increased hypoglycaemia or unexpected safety findings.
Keywords: exenatide once weekly; glucagon-like peptide-1 receptor agonist; insulin glargine; type 2 diabetes.
© 2018 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
C. G. served as the international coordinating investigator in the trial discussed in this paper, and has participated in scientific advisory boards of and has received consulting fees from Alfa Wasserman, AstraZeneca, Bayer AG, Berlin‐Chemie Mennarini, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk and Sanofi. J. P. F. has received research support from AbbVie, AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, IONIS, Janssen, Johnson and Johnson, Ligand, Merck, Mylan, Novartis, Novo Nordisk, Pfizer, Sanofi, Theracos and vTv Therapeutics, and has participated in scientific advisory boards of and has received consulting fees from AstraZeneca, Bristol‐Myers Squibb, Novo Nordisk, Sanofi and Theracos. A. S. has no conflicts of interest to report. S. J. has received consulting fees from AstraZeneca, Eli Lilly and Janssen. H. W. is a consultant for AstraZeneca. E. H. is an employee of and stockholder in AstraZeneca. J. R. has received research support from AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Hanmi, Intarcia, Janssen, Lexicon, Merck, Novo Nordisk, Pfizer and Sanofi, and has served on advisory boards of or received consulting honoraria from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Intarcia, Janssen, Novo Nordisk and Sanofi.
Figures
Similar articles
-
Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9).Diabetes Obes Metab. 2017 Jul;19(7):1024-1031. doi: 10.1111/dom.12937. Epub 2017 Apr 10. Diabetes Obes Metab. 2017. PMID: 28294499 Clinical Trial.
-
Safety and Efficacy of Exenatide Once Weekly Plus Dapagliflozin Once Daily Versus Exenatide or Dapagliflozin Alone in Patients With Type 2 Diabetes Inadequately Controlled With Metformin Monotherapy: 52-Week Results of the DURATION-8 Randomized Controlled Trial.Diabetes Care. 2018 Oct;41(10):2136-2146. doi: 10.2337/dc18-0680. Epub 2018 Aug 6. Diabetes Care. 2018. PMID: 30082326 Free PMC article. Clinical Trial.
-
Efficacy and tolerability of the new autoinjected suspension of exenatide once weekly versus exenatide twice daily in patients with type 2 diabetes.Diabetes Obes Metab. 2018 Jan;20(1):165-172. doi: 10.1111/dom.13056. Epub 2017 Aug 22. Diabetes Obes Metab. 2018. PMID: 28685973 Free PMC article. Clinical Trial.
-
Effects of exenatide twice daily, exenatide once weekly or insulin in patients with type 2 diabetes and baseline HbA1c ≥10.0%: Two pooled analyses including 20 randomised controlled trials.Int J Clin Pract. 2017 Dec;71(12). doi: 10.1111/ijcp.13029. Epub 2017 Oct 17. Int J Clin Pract. 2017. PMID: 29044860 Review.
-
[Twice-daily and weekly exenatide: clinical profile of two pioneer formulations in incretin therapy].Med Clin (Barc). 2014 Sep;143 Suppl 2:23-7. doi: 10.1016/S0025-7753(14)70105-8. Epub 2014 Oct 15. Med Clin (Barc). 2014. PMID: 25437462 Review. Spanish.
Cited by
-
A Comprehensive Review on Weight Loss Associated with Anti-Diabetic Medications.Life (Basel). 2023 Apr 14;13(4):1012. doi: 10.3390/life13041012. Life (Basel). 2023. PMID: 37109541 Free PMC article. Review.
-
Glycemic Efficacy, Weight Effects, and Safety of Once-Weekly Glucagon-Like Peptide-1 Receptor Agonists.J Manag Care Spec Pharm. 2018 Sep;24(9-a Suppl):S14-S29. doi: 10.18553/jmcp.2018.24.9-a.s14. J Manag Care Spec Pharm. 2018. PMID: 30156445 Free PMC article. Review.
-
Differential effects of GLP-1 receptor agonists on diabetic osteopathy in type 2 diabetes: a patient-stratified network meta-analysis.BMC Musculoskelet Disord. 2025 Aug 1;26(1):742. doi: 10.1186/s12891-025-09022-y. BMC Musculoskelet Disord. 2025. PMID: 40751153 Free PMC article.
-
Medicines for Obesity: Appraisal of Clinical Studies with Grading of Recommendations, Assessment, Development, and Evaluation Tool.Nutrients. 2023 Jan 24;15(3):606. doi: 10.3390/nu15030606. Nutrients. 2023. PMID: 36771314 Free PMC article.
-
Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials.Int J Mol Sci. 2023 Jun 21;24(13):10449. doi: 10.3390/ijms241310449. Int J Mol Sci. 2023. PMID: 37445623 Free PMC article. Review.
References
-
- American Diabetes Association . Standards of medical care in diabetes—2017: Classification and diagnosis of diabetes. Diabetes Care. 2017;40(suppl 1):S11‐S24. - PubMed
-
- Grandy S, Shaunik A, Hardy E. Effects of glucagon‐like peptide‐1 receptor agonists on beta‐cell function in patients with type 2 diabetes. J Diabetes Metab. 2016;7:643.
-
- American Diabetes Association . Standards of medical care in diabetes—2017: Pharmacologic approaches to glycemic treatment. Diabetes Care. 2017;40(suppl 1):S64‐S74. - PubMed
-
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 executive summary. Endocr Pract. 2017;23:207‐238. - PubMed
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140‐149. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

