Interventions for paracetamol (acetaminophen) overdose
- PMID: 29473717
- PMCID: PMC6491303
- DOI: 10.1002/14651858.CD003328.pub3
Interventions for paracetamol (acetaminophen) overdose
Abstract
Background: Paracetamol (acetaminophen) is the most widely used non-prescription analgesic in the world. Paracetamol is commonly taken in overdose either deliberately or unintentionally. In high-income countries, paracetamol toxicity is a common cause of acute liver injury. There are various interventions to treat paracetamol poisoning, depending on the clinical status of the person. These interventions include inhibiting the absorption of paracetamol from the gastrointestinal tract (decontamination), removal of paracetamol from the vascular system, and antidotes to prevent the formation of, or to detoxify, metabolites.
Objectives: To assess the benefits and harms of interventions for paracetamol overdosage irrespective of the cause of the overdose.
Search methods: We searched The Cochrane Hepato-Biliary Group Controlled Trials Register (January 2017), CENTRAL (2016, Issue 11), MEDLINE (1946 to January 2017), Embase (1974 to January 2017), and Science Citation Index Expanded (1900 to January 2017). We also searched the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov database (US National Institute of Health) for any ongoing or completed trials (January 2017). We examined the reference lists of relevant papers identified by the search and other published reviews.
Selection criteria: Randomised clinical trials assessing benefits and harms of interventions in people who have ingested a paracetamol overdose. The interventions could have been gastric lavage, ipecacuanha, or activated charcoal, or various extracorporeal treatments, or antidotes. The interventions could have been compared with placebo, no intervention, or to each other in differing regimens.
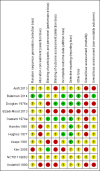
Data collection and analysis: Two review authors independently extracted data from the included trials. We used fixed-effect and random-effects Peto odds ratios (OR) with 95% confidence intervals (CI) for analysis of the review outcomes. We used the Cochrane 'Risk of bias' tool to assess the risks of bias (i.e. systematic errors leading to overestimation of benefits and underestimation of harms). We used Trial Sequential Analysis to control risks of random errors (i.e. play of chance) and GRADE to assess the quality of the evidence and constructed 'Summary of findings' tables using GRADE software.
Main results: We identified 11 randomised clinical trials (of which one acetylcysteine trial was abandoned due to low numbers recruited), assessing several different interventions in 700 participants. The variety of interventions studied included decontamination, extracorporeal measures, and antidotes to detoxify paracetamol's toxic metabolite; which included methionine, cysteamine, dimercaprol, or acetylcysteine. There were no randomised clinical trials of agents that inhibit cytochrome P-450 to decrease the activation of the toxic metabolite N-acetyl-p-benzoquinone imine.Of the 11 trials, only two had two common outcomes, and hence, we could only meta-analyse two comparisons. Each of the remaining comparisons included outcome data from one trial only and hence their results are presented as described in the trials. All trial analyses lack power to access efficacy. Furthermore, all the trials were at high risk of bias. Accordingly, the quality of evidence was low or very low for all comparisons. Interventions that prevent absorption, such as gastric lavage, ipecacuanha, or activated charcoal were compared with placebo or no intervention and with each other in one four-armed randomised clinical trial involving 60 participants with an uncertain randomisation procedure and hence very low quality. The trial presented results on lowering plasma paracetamol levels. Activated charcoal seemed to reduce the absorption of paracetamol, but the clinical benefits were unclear. Activated charcoal seemed to have the best risk:benefit ratio among gastric lavage, ipecacuanha, or supportive treatment if given within four hours of ingestion. There seemed to be no difference between gastric lavage and ipecacuanha, but gastric lavage and ipecacuanha seemed more effective than no treatment (very low quality of evidence). Extracorporeal interventions included charcoal haemoperfusion compared with conventional treatment (supportive care including gastric lavage, intravenous fluids, and fresh frozen plasma) in one trial with 16 participants. The mean cumulative amount of paracetamol removed was 1.4 g. One participant from the haemoperfusion group who had ingested 135 g of paracetamol, died. There were no deaths in the conventional treatment group. Accordingly, we found no benefit of charcoal haemoperfusion (very low quality of evidence). Acetylcysteine appeared superior to placebo and had fewer adverse effects when compared with dimercaprol or cysteamine. Acetylcysteine superiority to methionine was unproven. One small trial (low quality evidence) found that acetylcysteine may reduce mortality in people with fulminant hepatic failure (Peto OR 0.29, 95% CI 0.09 to 0.94). The most recent randomised clinical trials studied different acetylcysteine regimens, with the primary outcome being adverse events. It was unclear which acetylcysteine treatment protocol offered the best efficacy, as most trials were underpowered to look at this outcome. One trial showed that a modified 12-hour acetylcysteine regimen with a two-hour acetylcysteine 100 mg/kg bodyweight loading dose was associated with significantly fewer adverse reactions compared with the traditional three-bag 20.25-hour regimen (low quality of evidence). All Trial Sequential Analyses showed lack of sufficient power. Children were not included in the majority of trials. Hence, the evidence pertains only to adults.
Authors' conclusions: These results highlight the paucity of randomised clinical trials comparing different interventions for paracetamol overdose and their routes of administration and the low or very low level quality of the evidence that is available. Evidence from a single trial found activated charcoal seemed the best choice to reduce absorption of paracetamol. Acetylcysteine should be given to people at risk of toxicity including people presenting with liver failure. Further randomised clinical trials with low risk of bias and adequate number of participants are required to determine which regimen results in the fewest adverse effects with the best efficacy. Current management of paracetamol poisoning worldwide involves the administration of intravenous or oral acetylcysteine which is based mainly on observational studies. Results from these observational studies indicate that treatment with acetylcysteine seems to result in a decrease in morbidity and mortality, However, further evidence from randomised clinical trials comparing different treatments are needed.
Conflict of interest statement
AC: none known. CG: none known. JB: none known. NB: none known.
Figures
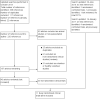






















Update of
-
Interventions for paracetamol (acetaminophen) overdose.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD003328. doi: 10.1002/14651858.CD003328.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2018 Feb 23;2:CD003328. doi: 10.1002/14651858.CD003328.pub3. PMID: 16625578 Updated.
References
References to studies included in this review
Arefi 2013 {published data only}
-
- Arefi M, Behnoush B, Pouraziz H, Yousefinejad V. Comparison of oral and intravenous N‐acetylcysteine administration in the treatment of acetaminophen poisoning. Scientific Journal of Kurdistan University of Medical Sciences 2013;18(2):36‐43. []
Bateman 2014 {published data only}
Douglas 1976a {published data only}
-
- Douglas AP, Hamlyn AN, James O. Controlled trial of cysteamine in treatment of acute paracetamol poisoning. Lancet 1976;2:111‐5. - PubMed
Eizadi‐Mood 2013 {published data only}
-
- Eizadi‐Mood N, Sabzghabaee AM, Siadat S, Yaraghi A, Shariati M, Gheshlaghi F, et al. Intravenous acetylcysteine versus oral and Intravenous acetylcysteine: does a combination therapy decrease side effects of acetylcysteine?. Pakistan Journal of Medical Sciences 2013;29(1 Suppl):308‐11. []
Gazzard 1974a {published data only}
Hamlyn 1981 {published data only}
-
- Hamlyn AN, Lesna M, Record CO, Smith PA, Path FRC, Watson AJ. Methionine and cysteamine in paracetamol overdose, prospective controlled trial of early therapy. Journal of International Medical Research 1981;9:226‐31. - PubMed
Hughes 1977 {published data only}
Keays 1991 {published data only}
Kerr 2005 {published data only}
-
- Kerr F, Dawson A, Whyte IM, Buckley N, Murray L, Graudins A, et al. The Australasian Clinical Toxicology Investigators Collaboration randomized trial of different loading infusion rates of N‐acetylcysteine. Annals of Emergency Medicine 2005;45(4):402‐8. [MEDLINE: ] - PubMed
NCT01118663 {published data only}
-
- Cumberland Pharmaceuticals. A multi‐center, double‐blind, randomized, controlled study to determine the efficacy and safety of a new formulation of acetylcysteine injection. clinicaltrials.gov/ct2/show/study/NCT01118663 Date first received: 4 August 2014.
References to studies excluded from this review
Bartels 2008 {published data only}
-
- Bartels S, Sivilotti M, Crosby D, Richard J. Are recommended doses of acetaminophen hepatotoxic for recently abstinent alcoholics? A randomized trial. Clinical Toxicology (Philadelphia, Pa.) 2008;46(3):243‐9. - PubMed
Bastaki 2006 {published data only}
-
- Bastaki SMA. Drugs update. Emirates Medical Journal 2006;24(3):245‐50. []
Buckley 1999b {published data only}
-
- Buckley NA, Whyte IM, O'Connell DL, Dawson AH. Oral or intravenous N‐acetylcysteine: which is the treatment of choice for acetaminophen (paracetamol) poisoning?. Journal of Toxicology. Clinical Toxicology 1999;37(6):759‐67. [; JC‐‐NLM: Journal ID:kan, 8213460] - PubMed
Burkhart 1995 {published data only}
-
- Burkhart KK, Janco N, Kulig KW, Rumack BH. Cimetidine as adjunctive treatment for acetaminophen overdose. Human & Experimental Toxicology 1995;14(3):299‐304. - PubMed
Clark 1996 {published data only}
-
- Clark RF, Chen R, Williams SR, Johnson CL, Harchelroad F. The use of ondansetron in the treatment of nausea and vomiting associated with acetaminophen poisoning. Journal of Toxicology. Clinical Toxicology 1996;34(2):163‐7. - PubMed
Cooper 2005 {published data only}
-
- Cooper GM, Couteur DG, Richardson D, Buckley NA. A randomized clinical trial of activated charcoal for the routine management of oral drug overdose. QJM : Monthly Journal of the Association of Physicians 2005;98(9):655‐60. [MEDLINE: ] - PubMed
Critchley 1983 {published data only}
-
- Critchley JA, Dyson EH, Scott AW, Jarvie DR, Prescott LF. Is there a place for cimetidine or ethanol in the treatment of paracetamol poisoning?. Lancet 1983;1(8338):1375‐6. - PubMed
Dordoni 1973 {published data only}
Douglas 1976b {published data only}
-
- Douglas AP, Hamlyn AN, James O. Controlled trial of cysteamine in treatment of acute paracetamol (acetaminophen) poisoning. Lancet 1976;1(7951):111‐5. - PubMed
Eguia 1997 {published data only}
-
- Eguia L, Materson BJ. Acetaminophen‐related acute renal failure without fulminant liver failure. Pharmacotherapy 1997;17(2):363‐70. - PubMed
Eyer 1991 {published data only}
-
- Eyer P, Sprenger M. Oral administration of activated charcoal‐sorbitol suspension as first aid in prevention of poison resorption?. Klinische Wochenschrift 1991;69(19):887‐94. - PubMed
Ferner 2001 {published data only}
-
- Ferner RE. Our poisoned patients. QJM : Monthly Journal of the Association of Physicians 2001;94(3):117‐20. - PubMed
Gawarammana 2006 {published data only}
-
- Gawarammana IB, Greene SL, Dargan PI, Jones AL. Australian clinical toxicology investigators collaboration randomized trial of different loading infusion rates of N‐acetylcysteine. Annals of Emergency Medicine 2006;47(1):124. - PubMed
Gazzard 1974b {published data only}
Gazzard 1975a {published data only}
-
- Gazzard BG, Hughes RD, Chhibber AD, Bennett JR, Murray‐Lyon IM, Dordoni B, et al. Proceedings: controlled trial of cysteamine and dimercaprol in the prevention of liver damage after paracetamol overdose. Gut 1975;16(10):839. - PubMed
Gazzard 1975b {published data only}
Guay 2003 {published data only}
-
- Guay DRP, Hajjar ER. Geriatric pharmacotherapy updates. American Journal Geriatric Pharmacotherapy 2003;1(2):96‐100.
Hamlyn 1980 {published data only}
-
- Hamlyn AN, Lesna M, Record CO, Smith PA, Watson AJ, Meredith T, et al. Prevention of hepatic necrosis in severe paracetamol (acetaminophen) poisoning: prospective controlled trial of early treatment with cysteamine or methionine [abstract]. Gut 1980;21(2):A448.
Hayes 2008 {published data only}
-
- Hayes BK. Frequency of medication errors with intravenous acetylcysteine for acetaminophen overdose. Annals of Pharmacotherapy 2008;42(6):776‐70. - PubMed
Hershkovitz 1996 {published data only}
-
- Hershkovitz E, Shorer Z, Levitas A, Tal A. Status epilepticus following intravenous N‐acetylcysteine therapy. Israel Journal of Medical Sciences 1996;32(11):1102‐4. [] - PubMed
Hughes 1976 {published data only}
-
- Hughes RD, Gazzard BG, Murray‐Lyon IM, Williams RS. The use of cysteamine and dimercaprol. Journal of International Medical Research 1976;4(4 Suppl):123‐9. [; CN‐00702516] - PubMed
Jalan 2006 {published data only}
-
- Jalan R, Williams R, Bernuau J. Paracetamol: are therapeutic doses entirely safe?. Lancet 2006;368(9554):2195‐6. - PubMed
Keays 1989 {published data only}
-
- Keays RT, Cove C, Forbes A, Alexander GJM, Williams R. Use of late N‐acetyl cysteine in severe paracetamol overdose [abstract]. Gut 1989;30(Suppl 10):A1512. [; CN‐00255461]
Koch 2010 {published data only}
-
- Koch A, Trautwein C. N‐acetylcysteine on its way to a broader application in patients with acute liver failure. Hepatology (Baltimore, Md.) 2010;51(1):338‐40. - PubMed
Kulig 1985 {published data only}
-
- Kulig K, Bar‐Or D, Cantrill SV, Rosen P, Rumack BH. Management of acutely poisoned patients without gastric emptying. Annals of Emergency Medicine 1985;14(6):562‐7. - PubMed
MacDonald 2006 {published data only}
-
- MacDonald TM. Acetaminophen: risk‐management urgently require. Pharmacoepidemiology and Drug Safety 2006;15(6):406‐9. - PubMed
Mann 1992 {published data only}
-
- Mann NS. Acetylcysteine for paracetamol‐induced hepatic failure. Annals of Internal Medicine 1992;116(Suppl 2):1992.
Mitchell 1984 {published data only}
Montoya‐Cabrera 1999 {published data only}
-
- Montoya‐Cabrera MA, Escalante‐Galindo P, Nava‐Juaez A, Terroba‐Larios VM, Teran‐Hernandez JA. Evaluation of the efficacy of N‐acetylcysteine administrated alone or in combination with activated charcoal in the treatment of acetaminophen overdose [Evaluacion de la eficacia de la N‐acetilcisteina administrada sola o conbinada con carbon activado en el tratamiento de la sobredosis por acetaminofen]. Gaceta Medica de Mexico 1999;135(3):239‐43. - PubMed
O'Grady 1988 {published data only}
-
- O'Grady JG, Gimson AE, O'Brien CJ, Pucknell A, Hughes RD, Williams R. Controlled trials of charcoal hemoperfusion and prognostic factors in fulminant hepatic failure. Gastroenterology 1988;94(5):1186‐92. - PubMed
Renzi 1985 {published data only}
-
- Renzi FP, Donovan JW, Martin TG. Concomitant use of activated charcoal and N‐acetylcysteine. Annals of Emergency Medicine 1985;14(6):568‐72. - PubMed
Saliba 2013 {published data only}
-
- Saliba F, Camus C, Durand F, Mathurin P, Letierce A, Delafosse B, et al. Albumin dialysis with a non cell artificial liver support device in patients with acute liver failure: a randomized, controlled trial. Annals of Internal Medicine 2013;159(8):522‐31. - PubMed
Spiller 2006 {published data only}
-
- Spiller HA, Winter ML, Klein‐Schwartz W, Bangh SA. Efficacy of activated charcoal administered more than four hours after acetaminophen overdose. Journal of Emergency Medicine 2006;30(1):1‐5. - PubMed
Additional references
Bailey 1998
-
- Bailey B, McGuigan MA. Management of anaphylactoid reactions to intravenous N‐acetylcysteine. Annals of Emergency Medicine 1998;31:710‐5. - PubMed
Begg 1994
-
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PUBMED: 7786990] - PubMed
Bernal 2013
-
- Bernal W, Hyyrylainen A, Gera A, Audimoolam VK, McPhail MJW, Auzinger G, et al. Lessons from look‐back in acute liver failure? A single centre experience of 3300 patients. Journal of Hepatology 2013;59:74‐80. - PubMed
Bradburn 2007
-
- Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta‐analytical methods with rare events. Statistics in Medicine 2007; Vol. 26, issue 1:53‐77. - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [PUBMED: 18411040] - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive. Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] - PubMed
Buckley 1999a
-
- Buckley NA, Whyte IM, O'Connell DL, Dawson AH. Activated charcoal reduces the need for N‐acetylcysteine treatment after acetaminophen (paracetamol) overdose. Journal of Toxicology. Clinical Toxicology 1999;37(6):753‐7. - PubMed
Buckley 2007
Cairney 2016
-
- Cairney DG, Beckwith HK, Al‐Hourani K, Eddleston M, Bateman DN, Dear JW. Plasma paracetamol concentration at hospital presentation has a dose‐dependent relationship with liver injury despite prompt treatment with intravenous acetylcysteine. Clinical Toxicology (Philadelphia, Pa.) 2016;54(5):405‐10. - PubMed
Chiew 2015
-
- Chiew AL, Fountain JS, Graudins A, Isbister GK, Reith D, Buckley NA. Summary statement: new guidelines for the management of paracetamol poisoning in Australia and New Zealand. Medical Journal of Australia 2015;203(5):215‐8. - PubMed
Chiew 2016
Chiew 2017
-
- Chiew AL, Isbister GK, Kirby KA, Page CB, Chan BSH, Buckley NA. Massive paracetamol overdose: an observational study of the effect of activated charcoal and increased acetylcysteine dose (ATOM‐2). Clinical Toxicology (Philadelphia, Pa.) 2017;55(10):1055‐65. - PubMed
Christophersen 2002
Chyka 2005
-
- Chyka PA, Seger D, Krenzelok EP, Vale JA, American Academy of Clinical Toxicology, European Association of Poisons Centres and Clinical Toxicologists. Position paper: single‐dose activated charcoal. Clinical Toxicology 2005;43(2):61‐87. [MEDLINE: ] - PubMed
Clark 1973
-
- Clark R, Borirakchanyavat V, Davidson AR, Thompson RP, Widdop B, Goulding R, et al. Hepatic damage and death from overdose of paracetamol. Lancet 1973;1(7794):66‐70. - PubMed
Daly 2008
-
- Daly FF, Fountain JS, Murray L, Graudins A, Buckley NA, Panel of Australian and New Zealand clinical toxicologists. Guidelines for the management of paracetamol poisoning in Australia and New Zealand ‐ explanation and elaboration. A consensus statement from clinical toxicologists consulting to the Australasian poisons information centres. Medical Journal of Australia 2008;188(5):296‐301. - PubMed
Dart 2006
-
- Dart R, Erdman A, Olson K, Christianson G, Manoguerra A, Chyka P, et al. Acetaminophen poisoning: an evidence‐based consensus guideline for out‐of‐hospital management. Clinical Toxicology 2006;44(1):1‐18. [] - PubMed
Davidson 1966
DeMets 1987
-
- DeMets DL. Practical aspects in monitoring: a brief review. Statistics in Medicine 1987;6(7):753‐60. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Doyon 2009
-
- Doyon S, Klein‐Schwartz W. Hepatotoxicity despite early administration of intravenous N‐acetylcysteine for acute acetaminophen overdose. Academic Emergency Medicine 2009;16(1):34‐9. - PubMed
Duffull 2013
Eddleston 2008
Egger 1997
Gluud 2017
-
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)) 2017, Issue 5. Art. No.: LIVER.
Gosselin 2014
-
- Gosselin S, Juurlink DN, Kielstein JT, Ghannoum M, Lavergne V, Nolin TD, EXTRIP Workgroup. Extracorporeal treatment for acetaminophen poisoning: recommendations from the EXTRIP workgroup. Clinical Toxicology (Philadelphia, Pa.) 2014;52(852):856‐67. - PubMed
Green 2001
-
- Green R, Grierson R, Sitar DS, Tenenbbein M. How long after drug ingestion is activated charcoal still effective?. Journal of Toxicology. Clinical Toxicology 2001;39(6):601‐5. - PubMed
Green 2013
Gunnell 1997
Guyatt 2008
Harbord 2006
-
- Harbord RM, Egger M, Sterne JAC. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. - PubMed
Harrison 1990
-
- Harrison PM, Keays R, Bray GP, Alexander GJ, Williams R. Improved outcome of paracetamol‐induced fulminant hepatic failure by late administration of acetylcysteine. Lancet 1990;335(8705):1572‐3. - PubMed
Heard 2014
-
- Heard K, Rumack BH, Green JL, Bucher‐Bartelson B, Heard S, Bronstein AC, et al. A single‐arm clinical trial of a 48‐hour intravenous N‐acetylcysteine protocol for treatment of acetaminophen poisoning. Clinical Toxicology 2014;52:512‐8. - PubMed
Heard 2017
-
- Heard K, Newton A. Paracetamol overdose. BMJ Best Practice: bestpractice.bmj.com last updated: 28 March 2017:1‐46.
Henry 1984
Higgins 1996
-
- Higgins RM, Goldsmith DJ, MacDiarmid‐Gordon A, Taberner D, Venning MC, Ackrill P. Treating paracetamol overdose by charcoal haemoperfusion and long‐hours high‐flux dialysis. QJM : Monthly Journal of the Association of Physicians 1996;89(4):297‐306. - PubMed
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. - PubMed
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011 Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. [Available from www.cochrane‐handbook.org.]
Jakobsen 2014
Jones 1998
-
- Jones AL. Mechanism of action and value of N acetylcysteine in the treatment of early and late acetaminophen poisoning: a critical review. Journal of Toxicology. Clinical Toxicology 1998;36:277‐85. - PubMed
Kao 2003
-
- Kao LW, Kirk MA, Furbee RB, Mehta NH, Skinner JR, Brizendine EJ. What is the rate of adverse events after oral N‐acetylcysteine administered by the intravenous route to patients with suspected acetaminophen poisoning?. Annals of Emergency Medicine 2003;42(6):741‐50. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [PUBMED: 11730399] - PubMed
Koch‐Weser 1976
-
- Koch‐Weser J. Acetaminophen. New England Journal of Medicine 1976;295(23):1297‐300. - PubMed
Krenzelok 2004
-
- Krenzelok EP, McGuigan M, Lheur P, Manoguerra AS. Position paper: Ipecac syrup. Journal of Toxicology. Clinical Toxicology 2004;42(2):133‐43. [MEDLINE: ] - PubMed
Kwan 1995
-
- Kwan D, Bartle WR, Walker SE. Abnormal serum transaminases following therapeutic doses of acetaminophen in the absence of known risk factors. Digestive Diseases and Sciences 1995;40(9):1951‐5. - PubMed
Lee 2004
-
- Lee WM. Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. Hepatology (Baltimore, Md.) 2004;40(1):6‐9. [MEDLINE: ] - PubMed
Lee 2011
-
- Lee WM, Larson AM, Todd Stravitz R. AASLD position paper: the management of acute liver failure: update 2011. www.aasld.org/practiceguidelines/Documents/AcuteLiverFailureUpdate2011.pdf (accessed June 2017).
Liisanantti 2003
-
- Liisanantti J, Kaukoranta P, Martikainen M, Ala‐Kokko T. Aspiration pneumonia following severe self‐poisoning. Resuscitation 2003;56(1):49‐53. [MEDLINE: ] - PubMed
Lundh 2017
Mant 1984
Marks 2017
McElhatton 1997
-
- McElhatton PR, Sullivan FM, Volans GN. Paracetamol overdose in pregnancy analysis of the outcomes of 300 cases referred to the Teratology Information Service. Reproductive Toxicology (Elmsford, N.Y.) 1997;11(1):85‐94. - PubMed
MHPRA 2012
-
- Medicines and Healthcare products Regulatory Agency. Treating paracetamol overdose with intravenous acetylcysteine: new guidance, 2012. www.gov.uk/drug‐safety‐update/treating‐paracetamol‐overdose‐with‐intrave... (accessed 20 May 2016).
Mitchell 1974
-
- Mitchell JR, Thorgeirrson SS, Potter WZ. Acetaminophen induced hepatic injury: protective role of glutathione in man and rationale for therapy. Clinical Pharmacology and Therapeutics 1974;16(4):676‐84. [] - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Morgan 2005
-
- Morgan O, Griffiths C, Majeed A. Impact of paracetamol pack size restrictions on poisoning from paracetamol in England and Wales: an observational study. Journal of Public Health 2005;27(1):19‐24. [MEDLINE: ] - PubMed
O'Grady 1997
-
- O'Grady JG. Paracetamol‐induced acute liver failure. Journal of Hepatology 1997;26 (Suppl):41‐6. - PubMed
Olsson 1988
-
- Olsson B, Johansson M, Gabrielsson J, Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N‐acetylcysteine. Journal of Clinical Pharmacology 1988;34(1):77‐82. - PubMed
Park 2015
Prescott 1973
Prescott 1976
-
- Prescott LF, Sutherland GR, Park J, Smith IJ, Proudfoot AT. Cysteamine, methionine, and penicillamine in the treatment of paracetamol poisoning. Lancet 1976;2(7977):109‐13. - PubMed
Prescott 1979
Prescott 1980
Prescott 2009
Reuben 2016
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Rumack 1975
-
- Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics 1975;55:871‐6. - PubMed
Rumack 2012
-
- Rumack BH, Bateman DN. Acetaminophen and acetylcysteine dose and duration: past, present and future. Clinical Toxicology 2012;50:91‐8. - PubMed
Sandilands 2009
-
- Sandilands EA, Bateman DN. Adverse reactions associated with acetylcysteine. Clinical Toxicology (Philadelphia, Pa.) 2009;47(2):81‐8. - PubMed
Savović 2012a
-
- Savović J, Jones HE, Altman DG, Harris RJ, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [PUBMED: 22945832] - PubMed
Savović 2012b
-
- Savović J, Jones H, Altman D, Harris R, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. [PUBMED: 22989478] - PubMed
Schmidt 2001
Schmidt 2002
-
- Schmidt LE, Knudsen TT, Dalhoff K, Bendtsen F. Effect of acetylcysteine on prothrombin index in paracetamol poisoning without hepatocellular injury. Lancet 2002;360(9340):1151‐2. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Senarathna 2012
Shiago 2011
-
- Shiago K, Watson I, Reidenberg MM. Application to change the status of methionine or N‐acetylcysteine on the model list, 2011. www.who.int/selection_medicines/committees/expert/18/applications/Delete... (accessed 20 May 2016).
Skoog 2015
-
- Skoog M, Saarimäki JM, Gluud C, Sheinin M, Erlendsson K, Aamdal S, et al. Transparency and Registration in Clinical Research in the Nordic Countries. Oslo (Norway): Nordic Trial Alliance, NordForsk, 2015.
Smilkstein 1988
-
- Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N‐acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). New England Journal of Medicine 1988;319(24):1557‐62. - PubMed
Smilkstein 1991
-
- Smilkstein MJ, Bronstein AC, Linden C, Augenstein WL, Kulig KW, Rumack BH. Acetaminophen overdose: a 48‐hour intravenous N‐acetylcysteine treatment protocol. Annals of Emergency Medicine 1991;20(10):1058‐63. - PubMed
Speeg 1995
-
- Speeg KV, Bay MK. Prevention and treatment of drug‐induced liver disease. Gastroenterology Clinics of North America 1995;24(4):1047‐64. - PubMed
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [PUBMED: 18824467] - PubMed
Thorlund 2010
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA), 2011. ctu.dk/tsa/files/tsa_manual.pdf (accessed 20 May 2016).
TSA 2011 [Computer program]
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Vale 1995
-
- Vale JA, Proudfoot AT. Paracetamol (acetaminophen) poisoning. Lancet 1995;346(8974):547‐52. - PubMed
Vale 2004
-
- Vale JA, Kulig K, American Academy of Clinical Toxicology, European Association of Poisons Centres and Clinical Toxicologists. Position statement: gastric lavage. Journal of Toxicology. Clinical Toxicology 2004;42(7):933‐43. [MEDLINE: ] - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] - PubMed
Wetterslev 2009
Wetterslev 2017
Whyte 2000
-
- Whyte IM, Buckley NA, Reith DM, Goodhew I, Seldon M, Dawson AH. Acetaminophen causes an increased international normalized ratio by reducing functional factor VII. Therapeutic Drug Monitoring 2000;22(6):742‐8. - PubMed
Williamson 2013
-
- Williamson K, Wahl MS, Mycyk MB. Direct comparison of 20‐hour IV, 36‐hour oral, and 72‐hour oral acetylcysteine for treatment of acute acetaminophen poisoning. American Journal of Therapeutics 2013;20(1):37‐40. - PubMed
Wolf 2007
-
- Wolf SJ, Heard K, Sloan EP, Jagoda AS. Clinical policy: critical issues in the management of patients presenting to the emergency department with acetaminophen overdose. Annals of Emergency Medicine 2007;50:292‐313. - PubMed
Woo 2000
-
- Woo OF, Mueller PD, Olson KR, Anderson IB, Kim SY. Shorter duration of oral N‐acetylcysteine therapy for acute acetaminophen overdose. Annals of Emergency Medicine 2000;35(4):363‐8. - PubMed
Wood 2008
Yeates 2000
References to other published versions of this review
Brok 2002
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

