Chemo- and Regioselective Lysine Modification on Native Proteins
- PMID: 29473744
- PMCID: PMC5880509
- DOI: 10.1021/jacs.7b12874
Chemo- and Regioselective Lysine Modification on Native Proteins
Abstract
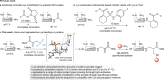
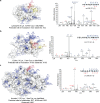
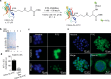
Site-selective chemical conjugation of synthetic molecules to proteins expands their functional and therapeutic capacity. Current protein modification methods, based on synthetic and biochemical technologies, can achieve site selectivity, but these techniques often require extensive sequence engineering or are restricted to the N- or C-terminus. Here we show the computer-assisted design of sulfonyl acrylate reagents for the modification of a single lysine residue on native protein sequences. This feature of the designed sulfonyl acrylates, together with the innate and subtle reactivity differences conferred by the unique local microenvironment surrounding each lysine, contribute to the observed regioselectivity of the reaction. Moreover, this site selectivity was predicted computationally, where the lysine with the lowest p Ka was the kinetically favored residue at slightly basic pH. Chemoselectivity was also observed as the reagent reacted preferentially at lysine, even in those cases when other nucleophilic residues such as cysteine were present. The reaction is fast and proceeds using a single molar equivalent of the sulfonyl acrylate reagent under biocompatible conditions (37 °C, pH 8.0). This technology was demonstrated by the quantitative and irreversible modification of five different proteins including the clinically used therapeutic antibody Trastuzumab without prior sequence engineering. Importantly, their native secondary structure and functionality is retained after the modification. This regioselective lysine modification method allows for further bioconjugation through aza-Michael addition to the acrylate electrophile that is generated by spontaneous elimination of methanesulfinic acid upon lysine labeling. We showed that a protein-antibody conjugate bearing a site-specifically installed fluorophore at lysine could be used for selective imaging of apoptotic cells and detection of Her2+ cells, respectively. This simple, robust method does not require genetic engineering and may be generally used for accessing diverse, well-defined protein conjugates for basic biology and therapeutic studies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

