Sulphated Flavonoids: Biosynthesis, Structures, and Biological Activities
- PMID: 29473839
- PMCID: PMC6017314
- DOI: 10.3390/molecules23020480
Sulphated Flavonoids: Biosynthesis, Structures, and Biological Activities
Abstract
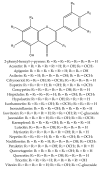
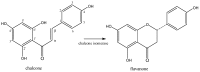
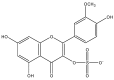
The great diversity of enzymatic reactions in plant secondary metabolism allows the continuous discovery of new natural compounds and derivatives. Flavonoids, for example, can be found as aglycone or as several sorts of glycosylated, acetylated, methylated, and sulphated derivatives. This review focuses on sulphated flavonoids, an uncommon group of flavonoid derivatives found in some plant families. This work presents a compilation of sulphated flavonoids and their natural sources reported in the literature. Biosynthetic aspects and biological activities have also been reviewed, showing that these particular kinds of natural compounds play an interesting role in plant metabolism, as well as being potential candidates for the development of new drugs.
Keywords: secondary metabolism; sulphated flavonoids; sulphation; sulphotransferases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources