Indispensable Role of Proteases in Plant Innate Immunity
- PMID: 29473858
- PMCID: PMC5855851
- DOI: 10.3390/ijms19020629
Indispensable Role of Proteases in Plant Innate Immunity
Abstract
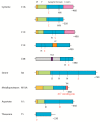
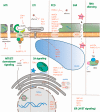
Plant defense is achieved mainly through the induction of microbe-associated molecular patterns (MAMP)-triggered immunity (MTI), effector-triggered immunity (ETI), systemic acquired resistance (SAR), induced systemic resistance (ISR), and RNA silencing. Plant immunity is a highly complex phenomenon with its own unique features that have emerged as a result of the arms race between plants and pathogens. However, the regulation of these processes is the same for all living organisms, including plants, and is controlled by proteases. Different families of plant proteases are involved in every type of immunity: some of the proteases that are covered in this review participate in MTI, affecting stomatal closure and callose deposition. A large number of proteases act in the apoplast, contributing to ETI by managing extracellular defense. A vast majority of the endogenous proteases discussed in this review are associated with the programmed cell death (PCD) of the infected cells and exhibit caspase-like activities. The synthesis of signal molecules, such as salicylic acid, jasmonic acid, and ethylene, and their signaling pathways, are regulated by endogenous proteases that affect the induction of pathogenesis-related genes and SAR or ISR establishment. A number of proteases are associated with herbivore defense. In this review, we summarize the data concerning identified plant endogenous proteases, their effect on plant-pathogen interactions, their subcellular localization, and their functional properties, if available, and we attribute a role in the different types and stages of innate immunity for each of the proteases covered.
Keywords: ETI; ISR; MTI; RNA silencing; SAR; plant immunity; plant proteases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

