Non-endocannabinoid N-acylethanolamines and 2-monoacylglycerols in the intestine
- PMID: 29473944
- PMCID: PMC6487557
- DOI: 10.1111/bph.14175
Non-endocannabinoid N-acylethanolamines and 2-monoacylglycerols in the intestine
Abstract
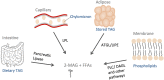
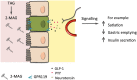
This review focuses on recent findings of the physiological and pharmacological role of non-endocannabinoid N-acylethanolamines (NAEs) and 2-monoacylglycerols (2-MAGs) in the intestine and their involvement in the gut-brain signalling. Dietary fat suppresses food intake, and much research concerns the known gut peptides, for example, glucagon-like peptide-1 (GLP-1) and cholecystokinin (CCK). NAEs and 2-MAGs represent another class of local gut signals most probably involved in the regulation of food intake. We discuss the putative biosynthetic pathways and targets of NAEs in the intestine as well as their anorectic role and changes in intestinal levels depending on the dietary status. NAEs can activate the transcription factor PPARα, but studies to evaluate the role of endogenous NAEs are generally lacking. Finally, we review the role of diet-derived 2-MAGs in the secretion of anorectic gut peptides via activation of GPR119. Both PPARα and GPR119 have potential as pharmacological targets for the treatment of obesity and the former for treatment of intestinal inflammation. LINKED ARTICLES: This article is part of a themed section on 8th European Workshop on Cannabinoid Research. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.10/issuetoc.
© 2018 The British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

