Alternaria alternata challenge at the nasal mucosa results in eosinophilic inflammation and increased susceptibility to influenza virus infection
- PMID: 29473965
- PMCID: PMC5992052
- DOI: 10.1111/cea.13123
Alternaria alternata challenge at the nasal mucosa results in eosinophilic inflammation and increased susceptibility to influenza virus infection
Abstract
Background: Eosinophils in the nasal mucosa are an elemental feature of allergic rhinitis.
Objective: Our objective was to explore eosinophilic inflammation and its impact on respiratory virus infection at the nasal mucosa.
Methods: Inflammation in the nasal mucosae of mice was evaluated in response to repetitive stimulation with strict intranasal volumes of a filtrate of Alternaria alternata. Mice were then challenged with influenza virus.
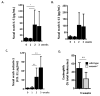
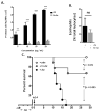
Results: Repetitive stimulation with A. alternata resulted in eosinophil recruitment to the nasal passages in association with elevated levels of IL-5, IL-13 and eotaxin-1; eosinophil recruitment was diminished in eotaxin-1-/- mice, and abolished in Rag1-/- mice. A. alternata also resulted in elevated levels of nasal wash IgA in both wild-type and eosinophil-deficient ∆dblGATA mice. Interestingly, A. alternata-treated mice responded to an influenza virus infection with profound weight loss and mortality compared to mice that received diluent alone (0% vs 100% survival, ***P < .001); the lethal response was blunted when A. alternata was heat-inactivated. Minimal differences in virus titre were detected, and eosinophils present in the nasal passages at the time of virus inoculation provided no protection against the lethal sequelae. Interestingly, nasal wash fluids from mice treated with A. alternata included more neutrophils and higher levels of pro-inflammatory mediators in response to virus challenge, among these, IL-6, a biomarker for disease severity in human influenza.
Conclusions and clinical relevance: Repetitive administration of A. alternata resulted in inflammation of the nasal mucosae and unanticipated morbidity and mortality in response to subsequent challenge with influenza virus. Interestingly, and in contrast to findings in the lower airways, eosinophils recruited to the nasal passages provided no protection against lethal infection. As increased susceptibility to influenza virus among individuals with rhinitis has been the subject of several clinical reports, this model may be used for further exploration of these observations.
Keywords: cytokine; eosinophil; inflammation; respiratory virus.
Published 2018. This article is a U.S. Government work and is in the public domain in the USA.
Figures







References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

