PharmGKB: A worldwide resource for pharmacogenomic information
- PMID: 29474005
- PMCID: PMC6002921
- DOI: 10.1002/wsbm.1417
PharmGKB: A worldwide resource for pharmacogenomic information
Abstract
As precision medicine becomes increasingly relevant in healthcare, the field of pharmacogenomics (PGx) also continues to gain prominence in the clinical setting. Leading institutions have begun to implement PGx testing and the amount of published PGx literature increases yearly. The Pharmacogenomics Knowledgebase (PharmGKB; www.pharmgkb.org) is one of the foremost worldwide resources for PGx knowledge, and the organization has been adapting and refocusing its mission along with the current revolution in genomic medicine. The PharmGKB website provides a diverse array of PGx information, from annotations of the primary literature to guidelines for adjusting drug treatment based on genetic information. It is freely available and accessible to everyone from researchers to clinicians to everyday citizens. PharmGKB was found over 17 years ago, but continues to be a vital resource for the entire PGx community and the general public. This article is categorized under: Translational, Genomic, and Systems Medicine > Translational Medicine.
Keywords: pharmGKB; pharmacodynamics; pharmacogenetics; pharmacogenomics; pharmacokinetics.
© 2018 The Authors. WIREs Systems Biology and Medicine published by Wiley Periodicals, Inc.
Figures
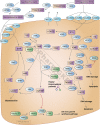


References
-
- Abbott, A. (2003). With your genes? Take one of these, three times a day. Nature, 425(6960), 760–762. - PubMed
-
- AstraZeneca Pharmaceuticals LP . (2015). IRESSA—gefitinib tablet, coated [package insert]. AstraZeneca Pharmaceuticals LP, Delaware.
-
- Berka, N. , Gill, J. M. , Liacini, A. , O'Bryan, T. , & Khan, F. M. (2012). Human leukocyte antigen (HLA) and pharmacogenetics: Screening for HLA‐B*57:01 among human immunodeficiency virus‐positive patients from southern Alberta. Human Immunology, 73(2), 164–167. - PubMed
-
- Caudle, K. E. , Klein, T. E. , Hoffman, J. M. , Muller, D. J. , Whirl‐Carrillo, M. , Gong, L. , … Johnson, S. G. (2014). Incorporation of pharmacogenomics into routine clinical practice: The clinical pharmacogenetics implementation consortium (CPIC) guideline development process. Current Drug Metabolism, 15(2), 209–217. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

