Different Conformational Subensembles of the Intrinsically Disordered Protein α-Synuclein in Cells
- PMID: 29474083
- PMCID: PMC5857923
- DOI: 10.1021/acs.jpclett.8b00092
Different Conformational Subensembles of the Intrinsically Disordered Protein α-Synuclein in Cells
Abstract
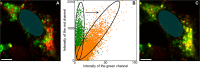
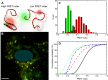
The intrinsically disordered protein α-synuclein (αS) is thought to play an important role in cellular membrane processes. Although in vitro experiments indicate that this initially disordered protein obtains structure upon membrane binding, NMR and EPR studies in cells could not single out any conformational subensemble. Here we microinjected small amounts of αS, labeled with a Förster resonance energy transfer (FRET) pair, into SH-SY5Y cells to investigate conformational changes upon membrane binding. Our FRET studies show a clear conformational difference between αS in the cytosol and when bound to small vesicles. The identification of these different conformational subensembles inside cells resolves the apparent contradiction between in vitro and in vivo experiments and shows that at least two different conformational subensembles of αS exist in cells. The existence of conformational subensembles supports the idea that αS can obtain different functions which can possibly be dynamically addressed with changing intracellular physicochemical conditions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Binolfi A.; Rasia R. M.; Bertoncini C. W.; Ceolin M.; Zweckstetter M.; Griesinger C.; Jovin T. M.; Fernandez C. O. Interaction of alpha-synuclein with divalent metal ions reveals key differences: A link between structure, binding specificity and fibrillation enhancement. J. Am. Chem. Soc. 2006, 128, 9893–9901. 10.1021/ja0618649. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

