Twenty-Five Years of Lamivudine: Current and Future Use for the Treatment of HIV-1 Infection
- PMID: 29474268
- PMCID: PMC5959256
- DOI: 10.1097/QAI.0000000000001660
Twenty-Five Years of Lamivudine: Current and Future Use for the Treatment of HIV-1 Infection
Abstract
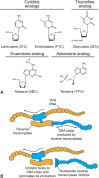
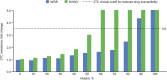
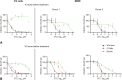
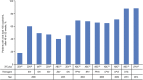
Innovation in medicine is a dynamic, complex, and continuous process that cannot be isolated to a single moment in time. Anniversaries offer opportunities to commemorate crucial discoveries of modern medicine, such as penicillin (1928), polio vaccination (inactivated, 1955; oral, 1961), the surface antigen of the hepatitis B virus (1967), monoclonal antibodies (1975), and the first HIV antiretroviral drugs (zidovudine, 1987). The advent of antiretroviral drugs has had a profound effect on the progress of the epidemiology of HIV infection, transforming a terminal, irreversible disease that caused a global health crisis into a treatable but chronic disease. This result has been driven by the success of antiretroviral drug combinations that include nucleoside reverse transcriptase inhibitors such as lamivudine. Lamivudine, an L-enantiomeric analog of cytosine, potently affects HIV replication by inhibiting viral reverse transcriptase enzymes at concentrations without toxicity against human polymerases. Although lamivudine was approved more than 2 decades ago, it remains a key component of first-line therapy for HIV because of its virological efficacy and ability to be partnered with other antiretroviral agents in traditional and novel combination therapies. The prominence of lamivudine in HIV therapy is highlighted by its incorporation in recent innovative treatment strategies, such as single-tablet regimens that address challenges associated with regimen complexity and treatment adherence and 2-drug regimens being developed to mitigate cumulative drug exposure and toxicities. This review summarizes how the pharmacologic and virologic properties of lamivudine have solidified its role in contemporary HIV therapy and continue to support its use in emerging therapies.
Figures
References
-
- EPIVIR [package insert]. Research Triangle Park, NC: ViiV Healthcare; 2013.
-
- EPIVIR-HBV [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
-
- World Health Organization (WHO). Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. In: 2nd ed WHO; 2016. Available at: http://apps.who.int/iris/bitstream/10665/208825/1/9789241549684_eng.pdf?.... Accessed January 31, 2018. - PubMed
-
- European AIDS Clinical Society. Guidelines. Version 9.0. EACS; 2017. Available at: http://www.eacsociety.org/files/guidelines_9.0-english.pdf. Accessed January 31, 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

