Contribution of S4 segments and S4-S5 linkers to the low-voltage activation properties of T-type CaV3.3 channels
- PMID: 29474447
- PMCID: PMC5825144
- DOI: 10.1371/journal.pone.0193490
Contribution of S4 segments and S4-S5 linkers to the low-voltage activation properties of T-type CaV3.3 channels
Abstract
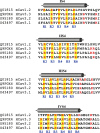
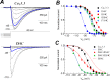
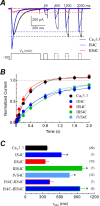
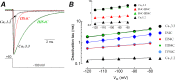
Voltage-gated calcium channels contain four highly conserved transmembrane helices known as S4 segments that exhibit a positively charged residue every third position, and play the role of voltage sensing. Nonetheless, the activation range between high-voltage (HVA) and low-voltage (LVA) activated calcium channels is around 30-40 mV apart, despite the high level of amino acid similarity within their S4 segments. To investigate the contribution of S4 voltage sensors for the low-voltage activation characteristics of CaV3.3 channels we constructed chimeras by swapping S4 segments between this LVA channel and the HVA CaV1.2 channel. The substitution of S4 segment of Domain II in CaV3.3 by that of CaV1.2 (chimera IIS4C) induced a ~35 mV shift in the voltage-dependence of activation towards positive potentials, showing an I-V curve that almost overlaps with that of CaV1.2 channel. This HVA behavior induced by IIS4C chimera was accompanied by a 2-fold decrease in the voltage-dependence of channel gating. The IVS4 segment had also a strong effect in the voltage sensing of activation, while substitution of segments IS4 and IIIS4 moved the activation curve of CaV3.3 to more negative potentials. Swapping of IIS4 voltage sensor influenced additional properties of this channel such as steady-state inactivation, current decay, and deactivation. Notably, Domain I voltage sensor played a major role in preventing CaV3.3 channels to inactivate from closed states at extreme hyperpolarized potentials. Finally, site-directed mutagenesis in the CaV3.3 channel revealed a partial contribution of the S4-S5 linker of Domain II to LVA behavior, with synergic effects observed in double and triple mutations. These findings indicate that IIS4 and, to a lesser degree IVS4, voltage sensors are crucial in determining the LVA properties of CaV3.3 channels, although the accomplishment of this function involves the participation of other structural elements like S4-S5 linkers.
Conflict of interest statement
Figures



Similar articles
-
Roles of molecular regions in determining differences between voltage dependence of activation of CaV3.1 and CaV1.2 calcium channels.J Biol Chem. 2004 Jun 25;279(26):26858-67. doi: 10.1074/jbc.M313981200. Epub 2004 Apr 20. J Biol Chem. 2004. PMID: 15100229
-
The effect of the outermost basic residues in the S4 segments of the Ca(V)3.1 T-type calcium channel on channel gating.Pflugers Arch. 2007 Dec;455(3):527-39. doi: 10.1007/s00424-007-0302-7. Epub 2007 Jul 19. Pflugers Arch. 2007. PMID: 17638012
-
Two distinct voltage-sensing domains control voltage sensitivity and kinetics of current activation in CaV1.1 calcium channels.J Gen Physiol. 2016 Jun;147(6):437-49. doi: 10.1085/jgp.201611568. Epub 2016 May 16. J Gen Physiol. 2016. PMID: 27185857 Free PMC article.
-
Biophysics and structure-function relationship of T-type Ca2+ channels.Cell Calcium. 2006 Aug;40(2):97-114. doi: 10.1016/j.ceca.2006.04.013. Epub 2006 Jun 13. Cell Calcium. 2006. PMID: 16777221 Review.
-
Characterization of the gating brake in the I-II loop of CaV3 T-type calcium channels.Channels (Austin). 2010 Nov-Dec;4(6):453-8. doi: 10.4161/chan.4.6.12889. Epub 2010 Nov 1. Channels (Austin). 2010. PMID: 21099341 Free PMC article. Review.
Cited by
-
Role of individual S4 segments in gating of Cav3.1 T-type calcium channel by voltage.Channels (Austin). 2018;12(1):378-387. doi: 10.1080/19336950.2018.1543520. Channels (Austin). 2018. PMID: 30403912 Free PMC article.
-
Increase of CaV3 channel activity induced by HVA β1b-subunit is not mediated by a physical interaction.BMC Res Notes. 2018 Nov 14;11(1):810. doi: 10.1186/s13104-018-3917-1. BMC Res Notes. 2018. PMID: 30428904 Free PMC article.
-
Calcium signaling in neurodevelopment and pathophysiology of autism spectrum disorders.Mol Biol Rep. 2022 Nov;49(11):10811-10823. doi: 10.1007/s11033-022-07775-6. Epub 2022 Jul 20. Mol Biol Rep. 2022. PMID: 35857176 Review.
References
-
- Berridge MJ, Bootman MD, Roderick HL (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529. doi: 10.1038/nrm1155 - DOI - PubMed
-
- Perez-Reyes E (2003) Molecular physiology of low-voltage-activated T-type calcium channels. Physiological Reviews 83: 117–161. doi: 10.1152/physrev.00018.2002 - DOI - PubMed
-
- Turner RW, Zamponi GW (2014) T-type channels buddy up. Pflugers Archiv-European Journal of Physiology 466: 661–675. doi: 10.1007/s00424-013-1434-6 - DOI - PMC - PubMed
-
- Lambert RC, Bessaih T, Crunelli V, Leresche N (2014) The many faces of T-type calcium channels. Pflugers Archiv-European Journal of Physiology 466: 415–423. doi: 10.1007/s00424-013-1353-6 - DOI - PubMed
-
- Weiss N, Zamponi GW (2013) Control of low-threshold exocytosis by T-type calcium channels. Biochimica Et Biophysica Acta-Biomembranes 1828: 1579–1586. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

