Distinct susceptibility of HIV vaccine vector-induced CD4 T cells to HIV infection
- PMID: 29474461
- PMCID: PMC5841825
- DOI: 10.1371/journal.ppat.1006888
Distinct susceptibility of HIV vaccine vector-induced CD4 T cells to HIV infection
Abstract
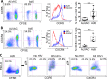
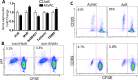
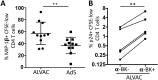
The concerns raised from adenovirus 5 (Ad5)-based HIV vaccine clinical trials, where excess HIV infections were observed in some vaccine recipients, have highlighted the importance of understanding host responses to vaccine vectors and the HIV susceptibility of vector-specific CD4 T cells in HIV vaccination. Our recent study reported that human Ad5-specific CD4 T cells induced by Ad5 vaccination (RV156A trial) are susceptible to HIV. Here we further investigated the HIV susceptibility of vector-specific CD4 T cells induced by ALVAC, a canarypox viral vector tested in the Thai trial RV144, as compared to Ad5 vector-specific CD4 T cells in the HVTN204 trial. We showed that while Ad5 vector-specific CD4 T cells were readily susceptible to HIV, ALVAC-specific CD4 T cells in RV144 PBMC were substantially less susceptible to both R5 and X4 HIV in vitro. The lower HIV susceptibility of ALVAC-specific CD4 T cells was associated with the reduced surface expression of HIV entry co-receptors CCR5 and CXCR4 on these cells. Phenotypic analyses identified that ALVAC-specific CD4 T cells displayed a strong Th1 phenotype, producing higher levels of IFN-γ and CCL4 (MIP-1β) but little IL-17. Of interest, ALVAC and Ad5 vectors induced distinct profiles of vector-specific CD8 vs. CD4 T-cell proliferative responses in PBMC, with ALVAC preferentially inducing CD8 T-cell proliferation, while Ad5 vector induced CD4 T-cell proliferation. Depletion of ALVAC-, but not Ad5-, induced CD8 T cells in PBMC led to a modest increase in HIV infection of vector-specific CD4 T cells, suggesting a role of ALVAC-specific CD8 T cells in protecting ALVAC-specific CD4 T cells from HIV. Taken together, our data provide strong evidence for distinct HIV susceptibility of CD4 T cells induced by different vaccine vectors and highlight the importance of better evaluating anti-vector responses in HIV vaccination.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- UNAIDS. Global AIDS Update 2016 Available from: http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016.
-
- Barouch DH. Novel adenovirus vector-based vaccines for HIV-1. Curr Opin HIV AIDS. 2010;5(5):386–90. doi: 10.1097/COH.0b013e32833cfe4c ; PubMed Central PMCID: PMC2967414. - DOI - PMC - PubMed
-
- Pantaleo G, Esteban M, Jacobs B, Tartaglia J. Poxvirus vector-based HIV vaccines. Curr Opin HIV AIDS. 2010;5(5):391–6. doi: 10.1097/COH.0b013e32833d1e87 . - DOI - PubMed
-
- Franchini G, Gurunathan S, Baglyos L, Plotkin S, Tartaglia J. Poxvirus-based vaccine candidates for HIV: two decades of experience with special emphasis on canarypox vectors. Expert Rev Vaccines. 2004;3(4 Suppl):S75–88. . - PubMed
-
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–93. Epub 2008/11/13. doi: 10.1016/S0140-6736(08)61591-3 ; PubMed Central PMCID: PMCPMC2721012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

