Clinical and genetic characterisation of dystrophin-deficient muscular dystrophy in a family of Miniature Poodle dogs
- PMID: 29474464
- PMCID: PMC5825102
- DOI: 10.1371/journal.pone.0193372
Clinical and genetic characterisation of dystrophin-deficient muscular dystrophy in a family of Miniature Poodle dogs
Abstract
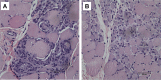
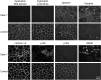
Four full-sibling intact male Miniature Poodles were evaluated at 4-19 months of age. One was clinically normal and three were affected. All affected dogs were reluctant to exercise and had generalised muscle atrophy, a stiff gait and a markedly elevated serum creatine kinase activity. Two affected dogs also showed poor development, learning difficulties and episodes of abnormal behaviour. In these two dogs, investigations into forebrain structural and metabolic diseases were unremarkable; electromyography demonstrated fibrillation potentials and complex repetitive discharges in the infraspinatus, supraspinatus and epaxial muscles. Histopathological, immunohistochemical and immunoblotting analyses of muscle biopsies were consistent with dystrophin-deficient muscular dystrophy. DNA samples were obtained from all four full-sibling male Poodles, a healthy female littermate and the dam, which was clinically normal. Whole genome sequencing of one affected dog revealed a >5 Mb deletion on the X chromosome, encompassing the entire DMD gene. The exact deletion breakpoints could not be experimentally ascertained, but we confirmed that this region was deleted in all affected males, but not in the unaffected dogs. Quantitative polymerase chain reaction confirmed all three affected males were hemizygous for the mutant X chromosome, while the wildtype chromosome was observed in the unaffected male littermate. The female littermate and the dam were both heterozygous for the mutant chromosome. Forty-four Miniature Poodles from the general population were screened for the mutation and were homozygous for the wildtype chromosome. The finding represents a naturally-occurring mutation causing dystrophin-deficient muscular dystrophy in the dog.
Conflict of interest statement
Figures




References
-
- Koenig M, Monaco AP, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53(2):219–28. . - PubMed
-
- Mendell JR, Shilling C, Leslie ND, Flanigan KM, al-Dahhak R, Gastier-Foster J, et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Annals of neurology. 2012;71(3):304–13. doi: 10.1002/ana.23528 . - DOI - PubMed
-
- Caskey CT, Nussbaum RL, Cohan LC, Pollack L. Sporadic occurrence of Duchenne muscular dystrophy: evidence for new mutation. Clin Genet. 1980;18(5):329–41. . - PubMed
-
- Nowak KJ, Davies KE. Duchenne muscular dystrophy and dystrophin: pathogenesis and opportunities for treatment. EMBO Rep. 2004;5(9):872–6. doi: 10.1038/sj.embor.7400221 ; PubMed Central PMCID: PMCPMC1299132. - DOI - PMC - PubMed
-
- Hoffman EP, Brown RH Jr., Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–28. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

