Lipidomic analysis of immune activation in equine leptospirosis and Leptospira-vaccinated horses
- PMID: 29474474
- PMCID: PMC5825116
- DOI: 10.1371/journal.pone.0193424
Lipidomic analysis of immune activation in equine leptospirosis and Leptospira-vaccinated horses
Abstract
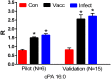
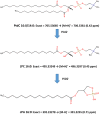
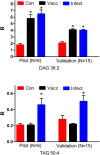
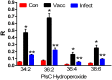
Currently available diagnostic assays for leptospirosis cannot differentiate vaccine from infection serum antibody. Several leptospiral proteins that are upregulated during infection have been described, but their utility as a diagnostic marker is still unclear. In this study, we undertook a lipidomics approach to determine if there are any differences in the serum lipid profiles of horses naturally infected with pathogenic Leptospira spp. and horses vaccinated against a commercially available bacterin. Utilizing a high-resolution mass spectrometry serum lipidomics analytical platform, we demonstrate that cyclic phosphatidic acids, diacylglycerols, and hydroperoxide oxidation products of choline plasmalogens are elevated in the serum of naturally infected as well as vaccinated horses. Other lipids of interest were triacylglycerols that were only elevated in the serum of infected horses and sphingomyelins that were increased only in the serum of vaccinated horses. This is the first report looking at the equine serum lipidome during leptospiral infection and vaccination.
Conflict of interest statement
Figures


Similar articles
-
Equine leptospirosis with some clinical observations.Ann Rech Vet. 1978;9(1):115-8. Ann Rech Vet. 1978. PMID: 707959
-
Detection of anti-Leptospira inhibitory antibodies in horses after vaccination.Microb Pathog. 2017 Sep;110:494-496. doi: 10.1016/j.micpath.2017.07.038. Epub 2017 Jul 25. Microb Pathog. 2017. PMID: 28754266
-
Serological study of leptospiral infections and endogenous uveitis among horses and ponies in the United Kingdom.Equine Vet J. 1987 Mar;19(2):125-8. doi: 10.1111/j.2042-3306.1987.tb02605.x. Equine Vet J. 1987. PMID: 3569193
-
Leptospirosis in horses.Vet Microbiol. 2013 Nov 29;167(1-2):61-6. doi: 10.1016/j.vetmic.2013.04.012. Epub 2013 Apr 16. Vet Microbiol. 2013. PMID: 23647816 Review.
-
Leptospirosis: An important infectious disease in North American horses.Equine Vet J. 2019 May;51(3):287-292. doi: 10.1111/evj.13069. Epub 2019 Jan 28. Equine Vet J. 2019. PMID: 30629756 Review.
Cited by
-
Comparison of anti-Leptospira antibodies by microscopic agglutination test in ruminants and equines of Urmia, Iran.Vet Res Forum. 2023;14(4):229-235. doi: 10.30466/vrf.2022.546475.3345. Epub 2023 Apr 15. Vet Res Forum. 2023. PMID: 37181853 Free PMC article.
-
2-Carba cyclic phosphatidic acid inhibits lipopolysaccharide-induced prostaglandin E2 production in a human macrophage cell line.Biochem Biophys Rep. 2019 Jul 19;19:100668. doi: 10.1016/j.bbrep.2019.100668. eCollection 2019 Sep. Biochem Biophys Rep. 2019. PMID: 31367683 Free PMC article.
-
Lipids in Equine Airway Inflammation: An Overview of Current Knowledge.Animals (Basel). 2024 Jun 18;14(12):1812. doi: 10.3390/ani14121812. Animals (Basel). 2024. PMID: 38929431 Free PMC article. Review.
-
Lipidomics of the chicken egg yolk: high-resolution mass spectrometric characterization of nutritional lipid families.Poult Sci. 2021 Feb;100(2):887-899. doi: 10.1016/j.psj.2020.11.020. Epub 2020 Nov 17. Poult Sci. 2021. PMID: 33518142 Free PMC article.
-
Systems vaccinology and big data in the vaccine development chain.Immunology. 2019 Jan;156(1):33-46. doi: 10.1111/imm.13012. Epub 2018 Nov 13. Immunology. 2019. PMID: 30317555 Free PMC article. Review.
References
-
- Adler B, de la Pena Moctezuma A. Leptospira and leptospirosis. Vet. Microbiol. 2010; 140:287–296. doi: 10.1016/j.vetmic.2009.03.012 . - DOI - PubMed
-
- Timoney J F, Kalimuthusamy N, Velineni S, Donahue J M, Artiushin S C, Fettinger M. A unique genotype of Leptospira interrogans serovar Pomona type kennewicki is associated with equine abortion. Vet Microbiol. 2011; 150: 349–353. doi: 10.1016/j.vetmic.2011.02.049 - DOI - PubMed
-
- Donahue J M, Smith B J, Poonacha K B, Donahoe J K, Rigsby C L. Prevalence and serovars of Leptospira involved in equine abortions in central Kentucky during the 1991–1993 foaling seasons. J. Vet. Diagn. Invest. 1995; 7:87–91. doi: 10.1177/104063879500700114 - DOI - PubMed
-
- Verma A, Stevenson B, Adler B. Leptospirosis in horses. Veterinary Microbiology 2013; 167: 61–66. doi: 10.1016/j.vetmic.2013.04.012 . - DOI - PubMed
-
- Verma A, Stevenson B. Leptospiral uveitis- there is more to it than meets the eye! Zoonoses Public Health, 2012;59 (Suppl. 2) 132–141. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

